Dicer in Schwann cells is required for myelination and axonal integrity
- PMID: 20463238
- PMCID: PMC6632556
- DOI: 10.1523/JNEUROSCI.0801-10.2010
Dicer in Schwann cells is required for myelination and axonal integrity
Abstract
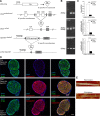
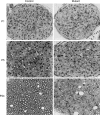
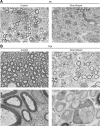
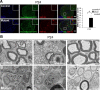
Dicer is responsible for the generation of mature micro-RNAs (miRNAs) and loading them into RNA-induced silencing complex (RISC). RISC functions as a probe that targets mRNAs leading to translational suppression and mRNA degradation. Schwann cells (SCs) in the peripheral nervous system undergo remarkable differentiation both in morphology and gene expression patterns throughout lineage progression to myelinating and nonmyelinating phenotypes. Gene expression in SCs is particularly tightly regulated and critical for the organism, as highlighted by the fact that a 50% decrease or an increase to 150% of normal gene expression of some myelin proteins, like PMP22, results in peripheral neuropathies. Here, we selectively deleted Dicer and consequently gene expression regulation by mature miRNAs from Mus musculus SCs. Our results show that in the absence of Dicer, most SCs arrest at the promyelinating stage and fail to start forming myelin. At the molecular level, the promyelinating transcription factor Krox20 and several myelin proteins [including myelin associated glycoprotein (MAG) and PMP22] were strongly reduced in mutant sciatic nerves. In contrast, the myelination inhibitors SOX2, Notch1, and Hes1 were increased, providing an additional potential basis for impaired myelination. A minor fraction of SCs, with some peculiar differences between sensory and motor fibers, overcame the myelination block and formed unusually thin myelin, in line with observed impaired neuregulin and AKT signaling. Surprisingly, we also found signs of axonal degeneration in Dicer mutant mice. Thus, our data indicate that miRNAs critically regulate Schwann cell gene expression that is required for myelination and to maintain axons via axon-glia interactions.
Figures



Comment in
-
Development: Schwann cells roll the dice.Nat Rev Neurosci. 2010 Jul;11(7):456-7. doi: 10.1038/nrn2873. Epub 2010 Jun 16. Nat Rev Neurosci. 2010. PMID: 21467990 No abstract available.
Similar articles
-
The deletion of dicer in mature myelinating glial cells causes progressive axonal degeneration but not overt demyelination in adult mice.Glia. 2018 Sep;66(9):1960-1971. doi: 10.1002/glia.23450. Epub 2018 May 4. Glia. 2018. PMID: 29726608
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
-
Akt Regulates Axon Wrapping and Myelin Sheath Thickness in the PNS.J Neurosci. 2016 Apr 20;36(16):4506-21. doi: 10.1523/JNEUROSCI.3521-15.2016. J Neurosci. 2016. PMID: 27098694 Free PMC article.
-
Ablation of Dicer from murine Schwann cells increases their proliferation while blocking myelination.PLoS One. 2010 Aug 27;5(8):e12450. doi: 10.1371/journal.pone.0012450. PLoS One. 2010. PMID: 20805985 Free PMC article.
-
How Schwann Cells Sort Axons: New Concepts.Neuroscientist. 2016 Jun;22(3):252-65. doi: 10.1177/1073858415572361. Epub 2015 Feb 16. Neuroscientist. 2016. PMID: 25686621 Free PMC article. Review.
Cited by
-
miR-381 Attenuates Peripheral Neuropathic Phenotype Caused by Overexpression of PMP22.Exp Neurobiol. 2019 Apr;28(2):279-288. doi: 10.5607/en.2019.28.2.279. Epub 2019 Apr 30. Exp Neurobiol. 2019. PMID: 31138995 Free PMC article.
-
Egr2-dependent microRNA-138 is dispensable for peripheral nerve myelination.Sci Rep. 2018 Feb 28;8(1):3817. doi: 10.1038/s41598-018-22010-8. Sci Rep. 2018. PMID: 29491350 Free PMC article.
-
MicroRNA and transcriptional crosstalk in myelinating glia.Neurochem Int. 2014 Nov;77:50-7. doi: 10.1016/j.neuint.2014.06.010. Epub 2014 Jun 27. Neurochem Int. 2014. PMID: 24979526 Free PMC article. Review.
-
Comparison of DNA Methylation in Schwann Cells before and after Peripheral Nerve Injury in Rats.Biomed Res Int. 2017;2017:5393268. doi: 10.1155/2017/5393268. Epub 2017 Mar 26. Biomed Res Int. 2017. PMID: 28459064 Free PMC article.
-
MicroRNAs modulate Schwann cell response to nerve injury by reinforcing transcriptional silencing of dedifferentiation-related genes.J Neurosci. 2011 Nov 30;31(48):17358-69. doi: 10.1523/JNEUROSCI.3931-11.2011. J Neurosci. 2011. PMID: 22131398 Free PMC article.
References
-
- Amaral PP, Dinger ME, Mercer TR, Mattick JS. The eukaryotic genome as an RNA machine. Science. 2008;319:1787–1789. - PubMed
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. - PubMed
-
- Bunge RP. Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Curr Opin Neurobiol. 1993;3:805–809. - PubMed
-
- Campana WM, Darin SJ, O'Brien JS. Phosphatidylinositol 3-kinase and Akt protein kinase mediate IGF-I- and prosaptide-induced survival in Schwann cells. J Neurosci Res. 1999;57:332–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
