Evidence for the involvement of Lfc and Tctex-1 in axon formation
- PMID: 20463241
- PMCID: PMC3845415
- DOI: 10.1523/JNEUROSCI.5420-09.2010
Evidence for the involvement of Lfc and Tctex-1 in axon formation
Abstract
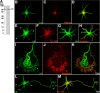
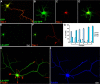
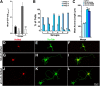
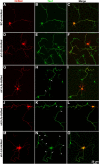
RhoA and Rac play key and opposite roles during neuronal polarization. We now show that Lfc, a guanosine nucleotide exchange factor (GEF), localizes to the Golgi apparatus and growth cones of developing neurons and negatively regulates neurite sprouting and axon formation through a Rho signaling pathway. Tctex-1, a dynein light chain implicated in axon outgrowth by modulating actin dynamics and Rac activity, colocalizes and physically interacts with Lfc, thus inhibiting its GEF activity, decreasing Rho-GTP levels, and functionally antagonizing Lfc during neurite formation.
Figures
Similar articles
-
Distinct roles for the two Rho GDP/GTP exchange factor domains of kalirin in regulation of neurite growth and neuronal morphology.J Neurosci. 2001 Nov 1;21(21):8426-34. doi: 10.1523/JNEUROSCI.21-21-08426.2001. J Neurosci. 2001. PMID: 11606631 Free PMC article.
-
A novel FERM domain including guanine nucleotide exchange factor is involved in Rac signaling and regulates neurite remodeling.J Neurosci. 2002 Oct 1;22(19):8504-13. doi: 10.1523/JNEUROSCI.22-19-08504.2002. J Neurosci. 2002. PMID: 12351724 Free PMC article.
-
Golgi-resident TRIO regulates membrane trafficking during neurite outgrowth.J Biol Chem. 2019 Jul 12;294(28):10954-10968. doi: 10.1074/jbc.RA118.007318. Epub 2019 May 31. J Biol Chem. 2019. PMID: 31152060 Free PMC article.
-
Involvement of p114-RhoGEF and Lfc in Wnt-3a- and dishevelled-induced RhoA activation and neurite retraction in N1E-115 mouse neuroblastoma cells.Mol Biol Cell. 2010 Oct 15;21(20):3590-600. doi: 10.1091/mbc.E10-02-0095. Epub 2010 Sep 1. Mol Biol Cell. 2010. PMID: 20810787 Free PMC article.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
Tctex-1, a novel interaction partner of Kidney Injury Molecule-1, is required for efferocytosis.J Cell Physiol. 2018 Oct;233(10):6877-6895. doi: 10.1002/jcp.26578. Epub 2018 Apr 25. J Cell Physiol. 2018. PMID: 29693725 Free PMC article.
-
Neurotoxicity of the pesticide rotenone on neuronal polarization: a mechanistic approach.Neural Regen Res. 2019 May;14(5):762-766. doi: 10.4103/1673-5374.249847. Neural Regen Res. 2019. PMID: 30688258 Free PMC article. Review.
-
Regulation of spine density and morphology by IQGAP1 protein domains.PLoS One. 2013;8(2):e56574. doi: 10.1371/journal.pone.0056574. Epub 2013 Feb 18. PLoS One. 2013. PMID: 23441206 Free PMC article.
-
Neuronal Polarity: Positive and Negative Feedback Signals.Front Cell Dev Biol. 2019 Apr 24;7:69. doi: 10.3389/fcell.2019.00069. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31069225 Free PMC article.
-
Changes in the GEF-H1 pathways after traumatic brain injury.J Neurotrauma. 2013 Aug 15;30(16):1449-56. doi: 10.1089/neu.2012.2673. Epub 2013 Jul 30. J Neurotrauma. 2013. PMID: 23611588 Free PMC article.
References
-
- Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786. - PubMed
-
- Arimura A, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. - PubMed
-
- Bhupinder P, Vohra S, Fu M, Heuckeroth RO. Protein kinase Czeta and glycogen synthase kinase-3beta control neuronal polarity in developing rodent enteric neurons, whereas SMAD-specific E3 ubiquitin protein ligase 1 promotes neurite growth but does not influence polarity. J Neurosci. 2007;27:9458–9468. - PMC - PubMed
-
- Birkenfeld J, Nalbant P, Yoon S, Bokoch G. Cellular functions of GEF-H1, a microtubule regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008;18:210–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
