Pathogenesis of alcoholic liver disease: the role of nuclear receptors
- PMID: 20463294
- PMCID: PMC3908670
- DOI: 10.1258/ebm.2009.009249
Pathogenesis of alcoholic liver disease: the role of nuclear receptors
Abstract
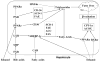
Ethanol consumption causes fatty liver, which can lead to inflammation, fibrosis, cirrhosis and even liver cancer. The molecular mechanisms by which ethanol exerts its damaging effects are extensively studied, but not fully understood. It is now evident that nuclear receptors (NRs), including retinoid x receptor alpha and peroxisome proliferator-activated receptors, play key roles in the regulation of lipid homeostasis and inflammation during the pathogenesis of alcoholic liver disease (ALD). Given their pivotal roles in physiological processes, NRs represent potential therapeutic targets for the treatment and prevention of numerous metabolic and lipid-related diseases including ALD. This review summarizes the factors that contribute to ALD and the molecular mechanisms of ALD with a focus on the role of NRs.
Figures

References
-
- Liu JD, Leung KW, Wang CK, Liao LY, Wang CS, Chen PH, Chen CC, Yeh EK. Alcohol-related problems in Taiwan with particular emphasis on alcoholic liver diseases. Alcohol Clin Exp Res. 1998;22:164S–9S. - PubMed
-
- Pares A, Caballeria J, Bruguera M, Torres M, Rodes J. Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. J Hepatol. 1986;2:33–42. - PubMed
-
- Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–13. - PubMed
-
- Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

