Proteome analysis of human aqueous humor
- PMID: 20463327
- PMCID: PMC3066620
- DOI: 10.1167/iovs.10-5531
Proteome analysis of human aqueous humor
Abstract
Purpose: Human aqueous humor (hAH) provides nutrition and immunity within the anterior chamber of the eye. Characterization of the protein composition of hAH will identify molecules involved in maintaining a homeostatic environment for anterior segment tissues. The present study was conducted to analyze the proteome of hAH.
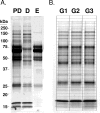
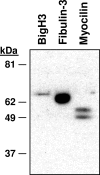
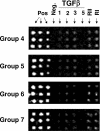
Methods: hAH samples obtained during elective cataract surgery were divided into three matched groups and immunodepleted of albumin, IgG, IgA, haploglobin, antitrypsin, and transferrin. Reduced and denatured proteins (20 μg) from each group were separated by gel electrophoresis. Thirty-three gel slices were excised from each of three gel lanes (n = 99), digested with trypsin, and subjected to nanoflow liquid chromatography electrospray ionization tandem mass spectrometry (nano-LC-ESI-MS/MS). The protein component of hAH was also analyzed by antibody-based protein arrays, and selected proteins were quantified.
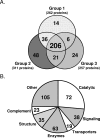
Results: A total of 676 proteins were identified in hAH. Of the 355 proteins identified by nano-LC-ESI-MS/MS, 206 were found in all three groups. Most of the proteins identified by nano-LC-ESI-MS/MS had catalytic, enzymatic, and structural properties. Using antibody-based protein arrays, 328 cytokines, chemokines, and receptors were identified. Most of the quantified proteins had concentrations that ranged between 0.1 and 2.5 ng/mL. Ten proteins were identified by both nano-LC-ESI-MS/MS and antibody protein arrays.
Conclusions: Proteomic analysis of hAH identified 676 nonredundant proteins. More than 80% of these proteins are novel identifications. The elucidation of the aqueous proteome will establish a foundation for protein function analysis and identification of differentially expressed markers associated with diseases of the anterior segment.
Figures
Similar articles
-
Proteomics of the aqueous humor in healthy New Zealand rabbits.Proteomics. 2007 Dec;7(23):4358-75. doi: 10.1002/pmic.200700300. Proteomics. 2007. PMID: 18040985
-
Anterior segment alterations and comparative aqueous humor proteomics in the buphthalmic rabbit (an American Ophthalmological Society thesis).Trans Am Ophthalmol Soc. 2011 Dec;109:66-114. Trans Am Ophthalmol Soc. 2011. PMID: 22253484 Free PMC article. Review.
-
Label-free LC-MS/MS quantitative analysis of aqueous humor from keratoconic and normal eyes.Mol Vis. 2015 Apr 25;21:451-60. eCollection 2015. Mol Vis. 2015. PMID: 25999673 Free PMC article.
-
Alterations in the aqueous humor proteome in patients with a glaucoma shunt device.Mol Vis. 2011;17:1891-900. Epub 2011 Jul 14. Mol Vis. 2011. PMID: 21850163 Free PMC article.
-
The Human Eye Proteome Project: Updates on an Emerging Proteome.Proteomics. 2018 Mar;18(5-6):e1700394. doi: 10.1002/pmic.201700394. Proteomics. 2018. PMID: 29356342 Free PMC article. Review.
Cited by
-
Comprehensive Proteomic Profiling of Aqueous Humor in Idiopathic Uveitis and Vogt-Koyanagi-Harada Syndrome.ACS Omega. 2024 Apr 15;9(16):18643-18653. doi: 10.1021/acsomega.3c10257. eCollection 2024 Apr 23. ACS Omega. 2024. PMID: 38680323 Free PMC article.
-
A Model of the Mechanisms Underpinning Unconventional Aqueous Humor Outflow.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):75. doi: 10.1167/iovs.66.4.75. Invest Ophthalmol Vis Sci. 2025. PMID: 40293397 Free PMC article.
-
The Juxtacanalicular Region of Ocular Trabecular Meshwork: A Tissue with a Unique Extracellular Matrix and Specialized Function.J Ocul Biol. 2013 Jun;1(1):3. J Ocul Biol. 2013. PMID: 24364042 Free PMC article.
-
Analysis of nestin protein in the aqueous humor as biomarker of open angle glaucoma.Heliyon. 2022 Jun 19;8(6):e09753. doi: 10.1016/j.heliyon.2022.e09753. eCollection 2022 Jun. Heliyon. 2022. PMID: 35789864 Free PMC article.
-
Aqueous humor proteomics analyzed by bioinformatics and machine learning in PDR cases versus controls.Clin Proteomics. 2024 May 19;21(1):36. doi: 10.1186/s12014-024-09481-w. Clin Proteomics. 2024. PMID: 38764026 Free PMC article.
References
-
- To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin Exp Optom. 2002;85:335–349 - PubMed
-
- Barsotti MF, Bartels SP, Freddo TF, Kamm RD. The source of protein in the aqueous humor of the normal monkey eye. Invest Ophthalmol Vis Sci. 1992;33:581–595 - PubMed
-
- Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990;31:125–137 - PubMed
-
- Freddo TF. The Glenn A. Fry Award Lecture 1992: aqueous humor proteins: a key for unlocking glaucoma? Optom Vis Sci. 1993;70:263–270 - PubMed
-
- McLaren JW, Ziai N, Brubaker RF. A simple three-compartment model of anterior segment kinetics. Exp Eye Res. 1993;56:355–366 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

