Podocytes require the engagement of cell surface heparan sulfate proteoglycans for adhesion to extracellular matrices
- PMID: 20463653
- PMCID: PMC3125125
- DOI: 10.1038/ki.2010.136
Podocytes require the engagement of cell surface heparan sulfate proteoglycans for adhesion to extracellular matrices
Abstract
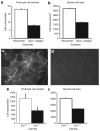
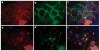
Podocytes adhere to the glomerular basement membrane by cell surface receptors. Since in other cells these adhesions are enhanced by cell surface proteoglycans, we examined the contribution of these molecules and their glycosaminoglycan side chains to podocyte adhesion by developing immortalized podocyte cell lines with (control) or without (mutant) heparan sulfate glycosaminoglycan chains. In adhesion assays control podocytes attached, spread, and migrated more efficiently compared with mutants, indicating a requirement for heparan sulfate chains in these processes. The proteoglycan syndecan-4 is known to have direct effects on cell attachment, spreading, and cytoskeletal organization. We found it localized to focal adhesions in control podocytes coincident with stress fiber formation. In mutant cells, syndecan-4 was associated with smaller focal contacts and cortical actin organization. Analysis by flow cytometry showed that mutant cells had twice the amount of surface syndecan-4 of control cells. Protein kinase Cα, a signaling molecule bound to and activated by syndecan-4, showed a fourfold increase in membrane localization-activation than that seen in control cells. In vivo, the loss of heparan sulfate glycosaminoglycans in PEXTKO mice led to a loss of glomerular syndecan-4. Overall, our study provides further evidence for a dynamic role of cell surface heparan sulfate glycosaminoglycans in podocyte activity.
Figures
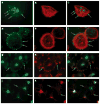
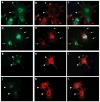
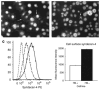
 , HS + cells stained for syndecan-4
, HS + cells stained for syndecan-4
 , and HS− cells stained for syndecan-4
, and HS− cells stained for syndecan-4
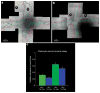
 . The chart shows the relative fluorescence intensity values for syndecan-4 staining in HS+ and HS− cells. The data show a net increase in the staining for syndecan-4 in HS− cells.
. The chart shows the relative fluorescence intensity values for syndecan-4 staining in HS+ and HS− cells. The data show a net increase in the staining for syndecan-4 in HS− cells.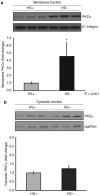

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

