Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging
- PMID: 20463658
- PMCID: PMC3055632
- DOI: 10.1038/npp.2010.68
Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging
Abstract
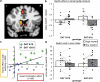
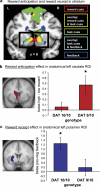
Dopamine has been hypothesized to provide the basis for the interaction between motivational and cognitive control. However, there is no evidence for this hypothesis in humans. We fill this gap by using fMRI, a novel behavioral paradigm and a common polymorphism in the DAT1 gene (SLC6A3). Carriers of the 9-repeat (9R) allele of a 40 base pair repeat polymorphism in the 3' untranslated region of DAT1, associated with high striatal dopamine, showed greater activity in the ventromedial striatum during reward anticipation than homozygotes for the 10-repeat allele, replicating previous genetic imaging studies. The crucial novel finding is that 9R carriers also exhibited a greater influence of anticipated reward on switch costs, as well as greater activity in the dorsomedial striatum during task switching in anticipation of high reward relative to low reward. These data establish a crucial role for human striatal dopamine in the modulation of cognitive flexibility by reward anticipation, thus, elucidating the neurochemical mechanism of the interaction between motivation and cognitive control.
Figures

References
-
- Aarts E, Roelofs A, van Turennout M. Attentional control of task and response in lateral and medial frontal cortex: brain activity and reaction time distributions. Neuropsychologia. 2009;47:2089–2099. - PubMed
-
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. - PubMed
-
- Bakshi VP, Kelley AE. Dopaminergic regulation of feeding behavior: II. Differential effects of amphetamine microinfusion into three striatal subregions. Psychobiology. 1991;19:233–242.
-
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

