Biophysics: Transporter in the spotlight
- PMID: 20463728
- PMCID: PMC2883250
- DOI: 10.1038/465171a
Biophysics: Transporter in the spotlight
Abstract
Membrane transporter proteins switch between conformational states to move substrates across membranes. The transition between these states can now be studied using single-molecule experiments.
Figures
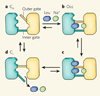
Comment on
-
Single-molecule dynamics of gating in a neurotransmitter transporter homologue.Nature. 2010 May 13;465(7295):188-93. doi: 10.1038/nature09057. Nature. 2010. PMID: 20463731 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

