Single-molecule dynamics of gating in a neurotransmitter transporter homologue
- PMID: 20463731
- PMCID: PMC2940119
- DOI: 10.1038/nature09057
Single-molecule dynamics of gating in a neurotransmitter transporter homologue
Abstract
Neurotransmitter:Na(+) symporters (NSS) remove neurotransmitters from the synapse in a reuptake process that is driven by the Na(+) gradient. Drugs that interfere with this reuptake mechanism, such as cocaine and antidepressants, profoundly influence behaviour and mood. To probe the nature of the conformational changes that are associated with substrate binding and transport, we have developed a single-molecule fluorescence imaging assay and combined it with functional and computational studies of the prokaryotic NSS homologue LeuT. Here we show molecular details of the modulation of intracellular gating of LeuT by substrates and inhibitors, as well as by mutations that alter binding, transport or both. Our direct observations of single-molecule transitions, reflecting structural dynamics of the intracellular region of the transporter that might be masked by ensemble averaging or suppressed under crystallographic conditions, are interpreted in the context of an allosteric mechanism that couples ion and substrate binding to transport.
Figures


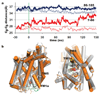
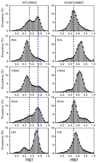
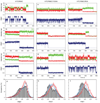
Comment in
-
Biophysics: Transporter in the spotlight.Nature. 2010 May 13;465(7295):171-2. doi: 10.1038/465171a. Nature. 2010. PMID: 20463728 Free PMC article.
References
-
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51(1–2):87–96. - PubMed
-
- Rudnick G. Mechanisms of biogenic amine neurotransmitter transporters. 2nd ed. Totowa, New Jersey: Humana Press Inc; 2002.
-
- Sonders MS, Quick M, Javitch JA. How did the neurotransmitter cross the bilayer? A closer view. Curr Opin Neurobiol. 2005;15(3):296–304. - PubMed
-
- Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269(10):7124–7130. - PubMed
-
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4(1):13–25. - PubMed
Methods References
-
- Schaffner W, Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973;56(2):502–514. - PubMed
-
- Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd ed. Baltimore, MD: Springer; 2006.
-
- Mujumdar RB, et al. Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug Chem. 1993;4(2):105–111. - PubMed
-
- Williams A, Winfield AS, Miller NJ. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst. 1983;108:1067–1071.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

