Besifloxacin: a novel anti-infective for the treatment of bacterial conjunctivitis
- PMID: 20463787
- PMCID: PMC2861926
- DOI: 10.2147/opth.s9604
Besifloxacin: a novel anti-infective for the treatment of bacterial conjunctivitis
Abstract
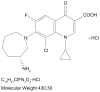
Bacterial conjunctivitis, commonly known as pink eye, is demographically unbiased in its prevalence and can be caused by a variety of aerobic and anaerobic bacteria. Timely empiric treatment with a broad-spectrum anti-infective, such as a topical fluoroquinolone, is critical in preventing potentially irreversible ocular damage. However, the rise in ocular methicillin- resistant Staphylococcus aureus isolates and the patterns of fluoroquinolone resistance for patients with other ocular bacterial infections mandate the need for new agents targeted for ocular use. Besifloxacin, a novel broad-spectrum fluoroquinolone, is approved for the treatment of bacterial conjunctivitis. It has a uniquely balanced dual-targeting activity that inhibits both DNA gyrase and topoisomerase IV and is associated with a lower incidence of resistance development. Besifloxacin is not marketed in other formulations, ensuring that its exposure is limited to bacterial populations in and around the eye. This specifically precludes any bacterial exposure to besifloxacin resulting from systemic use, which further reduces the likelihood of emergence of bacterial resistance. In vitro, besifloxacin has demonstrated equivalent or superior activity compared with other commonly used topical antibiotics. In clinical trials, besifloxacin has consistently demonstrated efficacy and safety in the treatment of patients with bacterial conjunctivitis. Besifloxacin is considered safe and is well tolerated with no observed contraindications.
Keywords: bacterial conjunctivitis; besifloxacin; besivance; conjunctivitis; fluoroquinolones.
Figures

References
-
- American Optometric Association. Care of the patient with conjunctivitis. Optometric clinical practice guideline. [Accessed September 22, 2009]. http://www.aoa.org/documents/CPG-11.pdf.
-
- Brook I. Ocular infections due to anaerobic bacteria in children. J Pediatr Ophthalmol Strabismus. 2008;45(2):78–84. - PubMed
-
- Tarabishy AB, Jeng BH. Bacterial conjunctivitis: a review for internists. Cleve Clin J Med. 2008;75(7):507–512. - PubMed
-
- Hussar DA. New drugs: golimumab, besifloxacin hydrochloride, and artemether/lumefantrine. J Am Pharm Assoc (2003) 2009;49(4):570–574. - PubMed
-
- Cavuoto K, Zutshi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008;115(1):51–56. - PubMed
LinkOut - more resources
Full Text Sources

