Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites
- PMID: 20463809
- PMCID: PMC2865532
- DOI: 10.1371/journal.ppat.1000877
Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites
Abstract
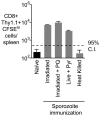
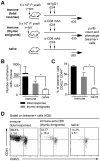
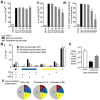
Immunization with irradiated sporozoites is currently the most effective vaccination strategy against liver stages of malaria parasites, yet the mechanisms underpinning the success of this approach are unknown. Here we show that the complete development of protective CD8+ T cell responses requires prolonged antigen presentation. Using TCR transgenic cells specific for the malaria circumsporozoite protein, a leading vaccine candidate, we found that sporozoite antigen persists for over 8 weeks after immunization--a remarkable finding since irradiated sporozoites are incapable of replication and do not differentiate beyond early liver stages. Persisting antigen was detected in lymphoid organs and depends on the presence of CD11c+ cells. Prolonged antigen presentation enhanced the magnitude of the CD8+ T cell response in a number of ways. Firstly, reducing the time primed CD8+ T cells were exposed to antigen in vivo severely reduced the final size of the developing memory population. Secondly, fully developed memory cells expanded in previously immunized mice but not when transferred to naïve animals. Finally, persisting antigen was able to prime naïve cells, including recent thymic emigrants, to become functional effector cells capable of eliminating parasites in the liver. Together these data show that the optimal development of protective CD8+ T cell immunity against malaria liver stages is dependent upon the prolonged presentation of sporozoite-derived antigen.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24:397–401. - PubMed
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. - PubMed
-
- Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, et al. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

