Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions
- PMID: 20463810
- PMCID: PMC2865522
- DOI: 10.1371/journal.ppat.1000880
Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions
Abstract
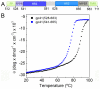
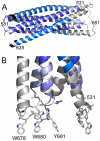
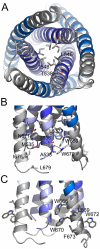
The HIV-1 envelope glycoprotein (Env) composed of the receptor binding domain gp120 and the fusion protein subunit gp41 catalyzes virus entry and is a major target for therapeutic intervention and for neutralizing antibodies. Env interactions with cellular receptors trigger refolding of gp41, which induces close apposition of viral and cellular membranes leading to membrane fusion. The energy released during refolding is used to overcome the kinetic barrier and drives the fusion reaction. Here, we report the crystal structure at 2 A resolution of the complete extracellular domain of gp41 lacking the fusion peptide and the cystein-linked loop. Both the fusion peptide proximal region (FPPR) and the membrane proximal external region (MPER) form helical extensions from the gp41 six-helical bundle core structure. The lack of regular coiled-coil interactions within FPPR and MPER splay this end of the structure apart while positioning the fusion peptide towards the outside of the six-helical bundle and exposing conserved hydrophobic MPER residues. Unexpectedly, the section of the MPER, which is juxtaposed to the transmembrane region (TMR), bends in a 90 degrees-angle sideward positioning three aromatic side chains per monomer for membrane insertion. We calculate that this structural motif might facilitate the generation of membrane curvature on the viral membrane. The presence of FPPR and MPER increases the melting temperature of gp41 significantly in comparison to the core structure of gp41. Thus, our data indicate that the ordered assembly of FPPR and MPER beyond the core contributes energy to the membrane fusion reaction. Furthermore, we provide the first structural evidence that part of MPER will be membrane inserted within trimeric gp41. We propose that this framework has important implications for membrane bending on the viral membrane, which is required for fusion and could provide a platform for epitope and lipid bilayer recognition for broadly neutralizing gp41 antibodies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. - PubMed
-
- Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, B R. The HIV Env-mediated fusion reaction. Biochimica Biophysica Acta. 2003;1614:36–50. - PubMed
-
- Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. - PubMed
-
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. - PubMed

