Accelerators, Brakes, and Gears of Actin Dynamics in Dendritic Spines
- PMID: 20463852
- PMCID: PMC2867483
- DOI: 10.2174/1874082000903020067
Accelerators, Brakes, and Gears of Actin Dynamics in Dendritic Spines
Abstract
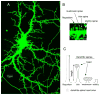
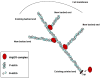
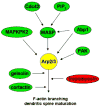
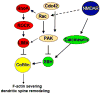
Dendritic spines are actin-rich structures that accommodate the postsynaptic sites of most excitatory synapses in the brain. Although dendritic spines form and mature as synaptic connections develop, they remain plastic even in the adult brain, where they can rapidly grow, change, or collapse in response to normal physiological changes in synaptic activity that underlie learning and memory. Pathological stimuli can adversely affect dendritic spine shape and number, and this is seen in neurodegenerative disorders and some forms of mental retardation and autism as well. Many of the molecular signals that control these changes in dendritic spines act through the regulation of filamentous actin (F-actin), some through direct interaction with actin, and others via downstream effectors. For example, cortactin, cofilin, and gelsolin are actin-binding proteins that directly regulate actin dynamics in dendritic spines. Activities of these proteins are precisely regulated by intracellular signaling events that control their phosphorylation state and localization. In this review, we discuss how actin-regulating proteins maintain the balance between F-actin assembly and disassembly that is needed to stabilize mature dendritic spines, and how changes in their activities may lead to rapid remodeling of dendritic spines.
Figures



Similar articles
-
Abl2:Cortactin Interactions Regulate Dendritic Spine Stability via Control of a Stable Filamentous Actin Pool.J Neurosci. 2021 Apr 7;41(14):3068-3081. doi: 10.1523/JNEUROSCI.2472-20.2021. Epub 2021 Feb 23. J Neurosci. 2021. PMID: 33622779 Free PMC article.
-
The structure and function of actin cytoskeleton in mature glutamatergic dendritic spines.Brain Res. 2014 Jul 21;1573:1-16. doi: 10.1016/j.brainres.2014.05.024. Epub 2014 May 20. Brain Res. 2014. PMID: 24854120 Review.
-
KLHL17/Actinfilin, a brain-specific gene associated with infantile spasms and autism, regulates dendritic spine enlargement.J Biomed Sci. 2020 Dec 1;27(1):103. doi: 10.1186/s12929-020-00696-1. J Biomed Sci. 2020. PMID: 33256713 Free PMC article.
-
Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity.J Neurosci. 2009 Jun 24;29(25):8129-42. doi: 10.1523/JNEUROSCI.4681-08.2009. J Neurosci. 2009. PMID: 19553453 Free PMC article.
-
Role of Drebrin in Synaptic Plasticity.Adv Exp Med Biol. 2017;1006:183-201. doi: 10.1007/978-4-431-56550-5_11. Adv Exp Med Biol. 2017. PMID: 28865021 Review.
Cited by
-
Cortactin-binding protein 2 modulates the mobility of cortactin and regulates dendritic spine formation and maintenance.J Neurosci. 2012 Jan 18;32(3):1043-55. doi: 10.1523/JNEUROSCI.4405-11.2012. J Neurosci. 2012. PMID: 22262902 Free PMC article.
-
Inhibition of coactivator-associated arginine methyltransferase 1 modulates dendritic arborization and spine maturation of cultured hippocampal neurons.J Biol Chem. 2017 Apr 14;292(15):6402-6413. doi: 10.1074/jbc.M117.775619. Epub 2017 Mar 6. J Biol Chem. 2017. PMID: 28264928 Free PMC article.
-
p140Cap regulates memory and synaptic plasticity through Src-mediated and citron-N-mediated actin reorganization.J Neurosci. 2014 Jan 22;34(4):1542-53. doi: 10.1523/JNEUROSCI.2341-13.2014. J Neurosci. 2014. PMID: 24453341 Free PMC article.
-
Medial orbitofrontal neurotrophin systems integrate hippocampal input into outcome-specific value representations.Cell Rep. 2022 Sep 13;40(11):111334. doi: 10.1016/j.celrep.2022.111334. Cell Rep. 2022. PMID: 36103822 Free PMC article.
-
Extracellular matrix protein laminin β1 regulates pain sensitivity and anxiodepression-like behaviors in mice.J Clin Invest. 2021 Aug 2;131(15):e146323. doi: 10.1172/JCI146323. J Clin Invest. 2021. PMID: 34156983 Free PMC article.
References
-
- Rao A, Craig AM. Signaling between the actin cytoskeleton and the postsynaptic density of dendritic spines. Hippocampus. 2000;10:527–41. - PubMed
-
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–11. - PubMed
-
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–8. - PubMed
-
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. - PubMed
-
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
