Spontaneous quaternary and tertiary T-R transitions of human hemoglobin in molecular dynamics simulation
- PMID: 20463873
- PMCID: PMC2865513
- DOI: 10.1371/journal.pcbi.1000774
Spontaneous quaternary and tertiary T-R transitions of human hemoglobin in molecular dynamics simulation
Abstract
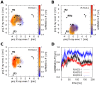
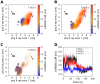
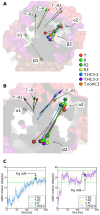
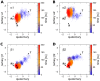
We present molecular dynamics simulations of unliganded human hemoglobin (Hb) A under physiological conditions, starting from the R, R2, and T state. The simulations were carried out with protonated and deprotonated HC3 histidines His(beta)146, and they sum up to a total length of 5.6 micros. We observe spontaneous and reproducible T-->R quaternary transitions of the Hb tetramer and tertiary transitions of the alpha and beta subunits, as detected from principal component projections, from an RMSD measure, and from rigid body rotation analysis. The simulations reveal a marked asymmetry between the alpha and beta subunits. Using the mutual information as correlation measure, we find that the beta subunits are substantially more strongly linked to the quaternary transition than the alpha subunits. In addition, the tertiary populations of the alpha and beta subunits differ substantially, with the beta subunits showing a tendency towards R, and the alpha subunits showing a tendency towards T. Based on the simulation results, we present a transition pathway for coupled quaternary and tertiary transitions between the R and T conformations of Hb.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Perutz MF, Wilkinson AJ, Paoli M, Dodson GG. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu Rev Biophys Biomol Struct. 1998;27:1–34. - PubMed
-
- Eaton WA, Henry ER, Hofrichter J, Bettati S, Viappiani C, et al. Evolution of allosteric models for hemoglobin. IUBMB Life. 2007;59:586–599. - PubMed
-
- Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. - PubMed
-
- Monod J, Wyman J, Changeux J-P. On the nature of Allosteric Transitions: A Plausible Model. J Mol Biol. 1965;12:88–118. - PubMed
-
- Shih T, Jones RT, Bonaventura J, Bonaventura C, Schneider RG. Involvement of His HC3 (146) beta in the Bohr effect of human hemoglobin. Studies of native and N-ethylmaleimide-treated hemoglobin A and hemoglobin Cowtown (beta 146 His replaced by Leu). J Biol Chem. 1984;259:967–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

