Optimal pandemic influenza vaccine allocation strategies for the Canadian population
- PMID: 20463898
- PMCID: PMC2865540
- DOI: 10.1371/journal.pone.0010520
Optimal pandemic influenza vaccine allocation strategies for the Canadian population
Abstract
Background: The world is currently confronting the first influenza pandemic of the 21(st) century. Influenza vaccination is an effective preventive measure, but the unique epidemiological features of swine-origin influenza A (H1N1) (pH1N1) introduce uncertainty as to the best strategy for prioritization of vaccine allocation. We sought to determine optimal prioritization of vaccine distribution among different age and risk groups within the Canadian population, to minimize influenza-attributable morbidity and mortality.
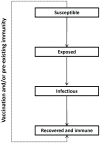
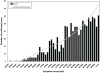
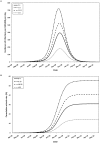
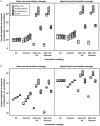
Methodology/principal findings: We developed a deterministic, age-structured compartmental model of influenza transmission, with key parameter values estimated from data collected during the initial phase of the epidemic in Ontario, Canada. We examined the effect of different vaccination strategies on attack rates, hospitalizations, intensive care unit admissions, and mortality. In all scenarios, prioritization of high-risk individuals (those with underlying chronic conditions and pregnant women), regardless of age, markedly decreased the frequency of severe outcomes. When individuals with underlying medical conditions were not prioritized and an age group-based approach was used, preferential vaccination of age groups at increased risk of severe outcomes following infection generally resulted in decreased mortality compared to targeting vaccine to age groups with higher transmission, at a cost of higher population-level attack rates. All simulations were sensitive to the timing of the epidemic peak in relation to vaccine availability, with vaccination having the greatest impact when it was implemented well in advance of the epidemic peak.
Conclusions/significance: Our model simulations suggest that vaccine should be allocated to high-risk groups, regardless of age, followed by age groups at increased risk of severe outcomes. Vaccination may significantly reduce influenza-attributable morbidity and mortality, but the benefits are dependent on epidemic dynamics, time for program roll-out, and vaccine uptake.
Conflict of interest statement
Figures
References
-
- Chan M. World now at the start of 2009 influenza pandemic. 2009. Available: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6.... Accessed 2009 Jul 26.
-
- National Advisory Committee on Immunization. Statement on influenza vaccination for the 2008–2009 season. An Advisory Committee Statement (ACS). Can Commun Dis Rep. 2008;34:1–46. - PubMed
-
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381. - PubMed
-
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–666. - PubMed
-
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

