Inhibition of chemokine-glycosaminoglycan interactions in donor tissue reduces mouse allograft vasculopathy and transplant rejection
- PMID: 20463901
- PMCID: PMC2865544
- DOI: 10.1371/journal.pone.0010510
Inhibition of chemokine-glycosaminoglycan interactions in donor tissue reduces mouse allograft vasculopathy and transplant rejection
Abstract
Background: Binding of chemokines to glycosaminoglycans (GAGs) is classically described as initiating inflammatory cell migration and creating tissue chemokine gradients that direct local leukocyte chemotaxis into damaged or transplanted tissues. While chemokine-receptor binding has been extensively studied during allograft transplantation, effects of glycosaminoglycan (GAG) interactions with chemokines on transplant longevity are less well known. Here we examine the impact of interrupting chemokine-GAG interactions and chemokine-receptor interactions, both locally and systemically, on vascular disease in allografts.
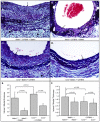
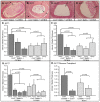
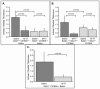
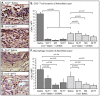
Methodology/principal findings: Analysis of GAG or CC chemokine receptor 2 (CCR2) deficiency were coupled with the infusion of viral chemokine modulating proteins (CMPs) in mouse aortic allograft transplants (n = 239 mice). Inflammatory cell invasion and neointimal hyperplasia were significantly reduced in N-deacetylase-N-sulfotransferase-1 (Ndst1(f/f)TekCre(+)) heparan sulfate (GAG)-deficient (Ndst1(-/-), p<0.044) and CCR2-deficient (Ccr2(-/-), p<0.04) donor transplants. Donor tissue GAG or CCR2 deficiency markedly reduced inflammation and vasculopathy, whereas recipient deficiencies did not. Treatment with three CMPs was also investigated; Poxviral M-T1 blocks CC chemokine receptor binding, M-T7 blocks C, CC, and CXC GAG binding, and herpesviral M3 binds receptor and GAG binding for all classes. M-T7 reduced intimal hyperplasia in wild type (WT) (Ccr2(+/+), p< or =0.003 and Ccr2(-/-), p</=0.027) aortic allografts, but not in Ndst1(-/-) aortic allografts (p = 0.933). M-T1 and M3 inhibited WT (Ccr2(+/+) and Ndst1(+/+), p< or =0.006) allograft vasculopathy, but did not block vasculopathy in Ccr2(-/-) (p = 0.61). M-T7 treatment alone, even without immunosuppressive drugs, also significantly prolonged survival of renal allograft transplants (p< or =0.001).
Conclusions/significance: Interruption of chemokine-GAG interactions, even in the absence of chemokine-receptor blockade, is a highly effective approach to reduction of allograft rejection, reducing vascular inflammation and prolonging allograft survival. Although chemokines direct both local and systemic cell migration, interruption of inherent chemokine responses in the donor tissue unexpectedly had a greater therapeutic impact on allograft vasculopathy.
Conflict of interest statement
Figures


Similar articles
-
Selective Deletion of Heparan Sulfotransferase Enzyme, Ndst1, in Donor Endothelial and Myeloid Precursor Cells Significantly Decreases Acute Allograft Rejection.Sci Rep. 2018 Sep 7;8(1):13433. doi: 10.1038/s41598-018-31779-7. Sci Rep. 2018. PMID: 30194334 Free PMC article.
-
Analysis of Chemokine-to-GAG Interactions in Model of Donor Renal Allograft Transplant.Methods Mol Biol. 2023;2597:25-38. doi: 10.1007/978-1-0716-2835-5_4. Methods Mol Biol. 2023. PMID: 36374412
-
Viral chemokine-binding proteins inhibit inflammatory responses and aortic allograft transplant vasculopathy in rat models.Transplantation. 2004 Jun 15;77(11):1652-60. doi: 10.1097/01.tp.0000131173.52424.84. Transplantation. 2004. PMID: 15201663
-
Targeting Chemokine-Glycosaminoglycan Interactions to Inhibit Inflammation.Front Immunol. 2020 Mar 31;11:483. doi: 10.3389/fimmu.2020.00483. eCollection 2020. Front Immunol. 2020. PMID: 32296423 Free PMC article. Review.
-
Chemokines and chemokine receptors in renal transplantation--from bench to bedside.Acta Physiol Hung. 2007 Mar;94(1-2):67-81. doi: 10.1556/APhysiol.94.2007.1-2.7. Acta Physiol Hung. 2007. PMID: 17444276 Review.
Cited by
-
Role of syndecan-1 in the interaction between dendritic cells and T cells.PLoS One. 2020 Jul 23;15(7):e0230835. doi: 10.1371/journal.pone.0230835. eCollection 2020. PLoS One. 2020. PMID: 32701966 Free PMC article.
-
Selective Deletion of Heparan Sulfotransferase Enzyme, Ndst1, in Donor Endothelial and Myeloid Precursor Cells Significantly Decreases Acute Allograft Rejection.Sci Rep. 2018 Sep 7;8(1):13433. doi: 10.1038/s41598-018-31779-7. Sci Rep. 2018. PMID: 30194334 Free PMC article.
-
Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function.Matrix Biol Plus. 2022 Sep 7;16:100121. doi: 10.1016/j.mbplus.2022.100121. eCollection 2022 Dec. Matrix Biol Plus. 2022. PMID: 36160687 Free PMC article. Review.
-
Virus-Derived Chemokine Modulating Protein Pre-Treatment Blocks Chemokine-Glycosaminoglycan Interactions and Significantly Reduces Transplant Immune Damage.Pathogens. 2022 May 16;11(5):588. doi: 10.3390/pathogens11050588. Pathogens. 2022. PMID: 35631109 Free PMC article.
-
Analysis of Chemokine-to-GAG Interactions in Model of Donor Renal Allograft Transplant.Methods Mol Biol. 2023;2597:25-38. doi: 10.1007/978-1-0716-2835-5_4. Methods Mol Biol. 2023. PMID: 36374412
References
-
- Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans - as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. - PubMed
-
- Cripps JG, Crespo FA, Romanovskis P, Spatola AF, Fenandez-Botran R. Modulation of acute inflammation by targeting glycosaminoglycan-cytokine interactions. Int Immunopharmacol. 2005;5:1622–1632. - PubMed
-
- Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nature Immunol. 2005;6:902–910. - PubMed
-
- Proudfoot AEI, Fritchley S, Borlat F, Shaw JP, Vilbois F, et al. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor sensitivity. J Biol Chem. 2001;276:10620–10626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

