On the control of acute rodent malaria infections by innate immunity
- PMID: 20463903
- PMCID: PMC2865546
- DOI: 10.1371/journal.pone.0010444
On the control of acute rodent malaria infections by innate immunity
Abstract
Does specific immunity, innate immunity or resource (red blood cell) limitation control the first peak of the blood-stage parasite in acute rodent malaria infections? Since mice deficient in specific immunity exhibit similar initial dynamics as wild-type mice it is generally viewed that the initial control of parasite is due to either limitation of resources (RBC) or innate immune responses. There are conflicting views on the roles of these two mechanisms as there is experimental evidence supporting both these hypotheses. While mathematical models based on RBC limitation are capable of describing the dynamics of primary infections, it was not clear whether a model incorporating the key features of innate immunity would be able to do the same. We examine the conditions under which a model incorporating parasite and innate immunity can describe data from acute Plasmodium chabaudi infections in mice. We find that innate immune response must decay slowly if the parasite density is to fall rather than equilibrate. Further, we show that within this framework the differences in the dynamics of two parasite strains are best ascribed to differences in susceptibility to innate immunity, rather than differences in the strains' growth rates or their propensity to elicit innate immunity. We suggest that further work is required to determine if innate immunity or resource limitation control acute malaria infections in mice.
Conflict of interest statement
Figures
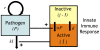
 , depends on two factors – its own replication (at rate
, depends on two factors – its own replication (at rate  ) and its clearance by activated innate immune cells
) and its clearance by activated innate immune cells  at rate
at rate  . The total number of innate immune cells equals
. The total number of innate immune cells equals  and they can be either in a resting or activated state. Since
and they can be either in a resting or activated state. Since  is the number of activated cells, the number of resting cells equals
is the number of activated cells, the number of resting cells equals  . Resting innate immune cells are activated at rate
. Resting innate immune cells are activated at rate  , and revert back to the inactive state at exponential rate
, and revert back to the inactive state at exponential rate  .
.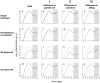
 (that is, no innate immunity is activated at infection),
(that is, no innate immunity is activated at infection),  day
day and
and  . Strain parameters are estimated using the single infection data from days 2–10 only (unshaded area). Strain parameters, I:
. Strain parameters are estimated using the single infection data from days 2–10 only (unshaded area). Strain parameters, I:  cells,
cells,  day
day ,
,  day
day ,
,  days
days cells
cells ,
,  day
day . II:
. II:  cells,
cells,  day
day ,
,  days
days cells
cells ,
,  days
days cells
cells ,
,  . III:
. III:  cells,
cells,  day
day ,
,  days
days cells
cells ,
,  day
day ,
,  day
day . The left-hand column shows the mean parasite counts over time from experimental data for single infections (panel a; n = 11 (AJ) and n = 14 (AS)) and co-infections (panels e, i, m; n = 4, 4 and 5 respectively).
. The left-hand column shows the mean parasite counts over time from experimental data for single infections (panel a; n = 11 (AJ) and n = 14 (AS)) and co-infections (panels e, i, m; n = 4, 4 and 5 respectively).References
-
- Borst P, Bitter W, McCulloch R, Van Leeuwen F, Rudenko G. Antigenic variation in malaria. Cell. 1995;82:1–4. - PubMed
-
- Molineaux L, Diebner HH, Eichner M, Collins WE, Jeffery GM, et al. Plasmodium falciparum parasitaemia described by a new mathematical model. Parasitology. 2001;122:379–91. - PubMed
-
- Paget-McNicol S, Gatton M, Hastings I, Saul A. The Plasmodium falciparum var gene switching rate, switching mechanism and patterns of parasite recrudescence described by mathematical modelling. Parasitology. 2002;124:225–35. - PubMed
-
- Recker M, Nee S, Bull PC, Kinyanjui S, Marsh K, et al. Transient cross-reactive immune responses can orchestrate antigenic variation in malaria. Nature. 2004;429:555–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

