Toxicology and drug delivery by cucurbit[n]uril type molecular containers
- PMID: 20463906
- PMCID: PMC2865549
- DOI: 10.1371/journal.pone.0010514
Toxicology and drug delivery by cucurbit[n]uril type molecular containers
Abstract
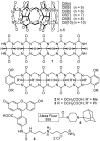
Background: Many drug delivery systems are based on the ability of certain macrocyclic compounds - such as cyclodextrins (CDs) - to act as molecular containers for pharmaceutical agents in water. Indeed beta-CD and its derivatives have been widely used in the formulation of hydrophobic pharmaceuticals despite their poor abilities to act as a molecular container (e.g., weak binding (K(a)<10(4) M(-1)) and their challenges toward chemical functionalization. Cucurbit[n]urils (CB[n]) are a class of molecular containers that bind to a variety of cationic and neutral species with high affinity (K(a)>10(4) M(-1)) and therefore show great promise as a drug delivery system.
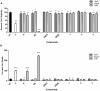
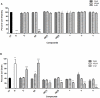
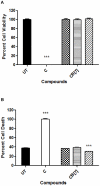
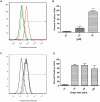
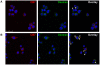
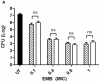
Methodology: In this study we investigated the toxicology, uptake, and bioactivity of two cucurbit[n]urils (CB[5] and CB[7]) and three CB[n]-type containers (Pentamer 1, methyl hexamer 2, and phenyl hexamer 3). All five containers demonstrated high cell tolerance at concentrations of up to 1 mM in cell lines originating from kidney, liver or blood tissue using assays for metabolic activity and cytotoxicity. Furthermore, the CB[7] molecular container was efficiently internalized by macrophages indicating their potential for the intracellular delivery of drugs. Bioactivity assays showed that the first-line tuberculosis drug, ethambutol, was as efficient in treating mycobacteria infected macrophages when loaded into CB[7] as when given in the unbound form. This result suggests that CB[7]-bound drug molecules can be released from the container to find their intracellular target.
Conclusion: Our study reveals very low toxicity of five members of the cucurbit[n]uril family of nanocontainers. It demonstrates the uptake of containers by cells and intracellular release of container-loaded drugs. These results provide initial proof-of-concept towards the use of CB[n] molecular containers as an advanced drug delivery system.
Conflict of interest statement
Figures
Similar articles
-
Paclitaxel interaction with cucurbit [7]uril and acyclic Cucurbit[4]uril nanocontainers: A computational approach.J Mol Graph Model. 2019 Jul;90:210-218. doi: 10.1016/j.jmgm.2019.05.010. Epub 2019 May 11. J Mol Graph Model. 2019. PMID: 31103913
-
Toxicity of cucurbit[7]uril and cucurbit[8]uril: an exploratory in vitro and in vivo study.Org Biomol Chem. 2010 May 7;8(9):2037-42. doi: 10.1039/b925555a. Epub 2010 Feb 17. Org Biomol Chem. 2010. PMID: 20401379
-
Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals.Nat Chem. 2012 Apr 15;4(6):503-10. doi: 10.1038/nchem.1326. Nat Chem. 2012. PMID: 22614387
-
Supramolecular Chemotherapy with Cucurbit[n]urils as Encapsulating Hosts.ACS Appl Bio Mater. 2023 Jun 19;6(6):2089-2101. doi: 10.1021/acsabm.3c00244. Epub 2023 May 24. ACS Appl Bio Mater. 2023. PMID: 37224296 Review.
-
Cucurbit[7]uril: an emerging candidate for pharmaceutical excipients.Ann N Y Acad Sci. 2017 Jun;1398(1):108-119. doi: 10.1111/nyas.13376. Ann N Y Acad Sci. 2017. PMID: 28692768 Review.
Cited by
-
Host-guest interaction of cucurbit[8]uril with oroxin A and its effect on the properties of oroxin A.Beilstein J Org Chem. 2020 Sep 22;16:2332-2337. doi: 10.3762/bjoc.16.194. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 33029251 Free PMC article.
-
Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins.Pharmaceutics. 2019 Feb 21;11(2):91. doi: 10.3390/pharmaceutics11020091. Pharmaceutics. 2019. PMID: 30795556 Free PMC article. Review.
-
Protocols utilizing constant pH molecular dynamics to compute pH-dependent binding free energies.J Phys Chem B. 2015 Jan 22;119(3):861-72. doi: 10.1021/jp505777n. Epub 2014 Aug 25. J Phys Chem B. 2015. PMID: 25134690 Free PMC article.
-
HYDROPHOBE Challenge: A Joint Experimental and Computational Study on the Host-Guest Binding of Hydrocarbons to Cucurbiturils, Allowing Explicit Evaluation of Guest Hydration Free-Energy Contributions.J Phys Chem B. 2017 Dec 14;121(49):11144-11162. doi: 10.1021/acs.jpcb.7b09175. Epub 2017 Dec 1. J Phys Chem B. 2017. PMID: 29140701 Free PMC article.
-
Overcoming barriers with non-covalent interactions: supramolecular recognition of adamantyl cucurbit[n]uril assemblies for medical applications.RSC Med Chem. 2023 Dec 6;15(2):433-471. doi: 10.1039/d3md00596h. eCollection 2024 Feb 21. RSC Med Chem. 2023. PMID: 38389878 Free PMC article. Review.
References
-
- Peck RW. Driving earlier clinical attrition: if you want to find the needle, burn down the haystack. Considerations for biomarker development. Drug Discovery Today. 2007;12:289–294. - PubMed
-
- Verma RK, Krishna DM, Garg S. Formulation aspects in the development of osmotically controlled oral drug delivery systems. Journal of Controlled Release. 2002;79:7–27. - PubMed
-
- van de Waterbeemd HL, Artursson Per. Mannhold RaimundHK, Folkers Gerd., editors. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability (Methods and Principles in Medicinal Chemistry); 2003. 602. Wiley, John & Sons, Incorporated.
-
- Samad A, Alam MI, Saxena K. Dendrimers: A Class of Polymers in the Nanotechnology for the Delivery of Active Pharmaceuticals. Current Pharmaceutical Design. 2009;15:2958–2969. - PubMed
-
- Spataro G, Malecaze F, Turrin CO, Soler V, Duhayon C, et al. Designing dendrimers for ocular drug delivery. Eur J Med Chem 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

