CFTR depletion results in changes in fatty acid composition and promotes lipogenesis in intestinal Caco 2/15 cells
- PMID: 20463919
- PMCID: PMC2864762
- DOI: 10.1371/journal.pone.0010446
CFTR depletion results in changes in fatty acid composition and promotes lipogenesis in intestinal Caco 2/15 cells
Erratum in
- PLoS One. 2010;5(5). doi: 10.1371/annotation/f9b8a9d2-4be3-4981-92f4-a3b4cb0b0bf5
Abstract
Background: Abnormal fatty acid composition (FA) in plasma and tissue lipids frequently occurs in homozygous and even in heterozygous carriers of cystic fibrosis transmembrane conductance regulator (CFTR) mutations. The mechanism(s) underlying these abnormalities remained, however, poorly understood despite the potentially CFTR contributing role.
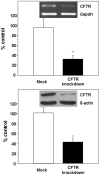
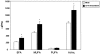
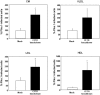
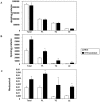
Methodology/principal findings: The aim of the present study was to investigate the impact of CFTR depletion on FA uptake, composition and metabolism using the intestinal Caco-2/15 cell line. shRNA-mediated cftr gene silencing induced qualitative and quantitative modifications in FA composition in differentiated enterocytes as determined by gas-liquid chromatography. With the cftr gene disruption, there was a 1,5 fold increase in the total FA amount, largely attributable to monounsaturated and saturated FA compared to controls. The activity of delta-7 desaturase, estimated by the 16:1(n-7)/16:0, was significantly higher in knockdown cells and consistent with the striking elevation of the n-7 FA family. When incubated with [14C]-oleic acid, CFTR-depleted cells were capable of quick incorporation and export to the medium concomitantly with the high protein expression of L-FABP known to promote intracellular FA trafficking. Accordingly, lipoprotein vehicles (CM, VLDL, LDL and HDL), isolated from CFTR knockdown cells, exhibited higher levels of radiolabeled FA. Moreover, in the presence of [14C]-acetate, knockdown cells exhibited enhanced secretion of newly synthesized phospholipids, triglycerides, cholesteryl esters and free FA, thereby suggesting a stimulation of the lipogenic pathway. Conformably, gene expression of SREBP-1c, a key lipogenic transcription factor, was increased while protein expression of the phosphorylated and inactive form of acetylCoA carboxylase was reduced, confirming lipogenesis induction. Finally, CFTR-depleted cells exhibited lower gene expression of transcription factors (PPARalpha, LXRalpha, LXRbeta and RXRalpha).
Conclusions/significance: Collectively, our results indicate that CFTR depletion may disrupt FA homeostasis in intestinal cells through alterations in FA uptake and transport combined with stimulation of lipogenesis that occurs by an LXR/RXR-independent mechanism. These findings exclude a contributing role of CFTR in CF-associated fat malabsorption.
Conflict of interest statement
Figures









Similar articles
-
CFTR knockdown stimulates lipid synthesis and transport in intestinal Caco-2/15 cells.Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297(6):G1239-49. doi: 10.1152/ajpgi.00206.2009. Epub 2009 Oct 1. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19808659
-
CFTR Deletion Confers Mitochondrial Dysfunction and Disrupts Lipid Homeostasis in Intestinal Epithelial Cells.Nutrients. 2018 Jun 27;10(7):836. doi: 10.3390/nu10070836. Nutrients. 2018. PMID: 29954133 Free PMC article.
-
Increased lipogenesis and stearate accelerate vascular calcification in calcifying vascular cells.J Biol Chem. 2011 Jul 8;286(27):23938-49. doi: 10.1074/jbc.M111.237065. Epub 2011 May 19. J Biol Chem. 2011. PMID: 21596756 Free PMC article.
-
Mechanisms of regulation of gene expression by fatty acids.Lipids. 2004 Nov;39(11):1077-83. doi: 10.1007/s11745-004-1333-0. Lipids. 2004. PMID: 15726822 Review.
-
Fatty acid metabolism: The crossroads in intestinal homeostasis and tumor.Metabolism. 2025 Aug;169:156273. doi: 10.1016/j.metabol.2025.156273. Epub 2025 Apr 23. Metabolism. 2025. PMID: 40280478 Review.
Cited by
-
Impaired intestinal free fatty acid transport followed by chylomicron malformation, not pancreatic insufficiency, cause metabolic defects in cystic fibrosis.J Lipid Res. 2024 Jul;65(7):100551. doi: 10.1016/j.jlr.2024.100551. Epub 2024 Jul 13. J Lipid Res. 2024. PMID: 39002195 Free PMC article.
-
Acetyl-CoA carboxylase inhibition regulates microtubule dynamics and intracellular transport in cystic fibrosis epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2019 Jun 1;316(6):L1081-L1093. doi: 10.1152/ajplung.00369.2018. Epub 2019 Mar 20. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30892081 Free PMC article.
-
High-fat diets affect energy and bone metabolism in growing rats.Eur J Nutr. 2012 Jun;51(4):399-406. doi: 10.1007/s00394-011-0223-2. Epub 2011 Jul 2. Eur J Nutr. 2012. PMID: 21725629
-
2,3,7,8-Tetrachlorodibenzo-p-Dioxin Alters Lipid Metabolism and Depletes Immune Cell Populations in the Jejunum of C57BL/6 Mice.Toxicol Sci. 2015 Dec;148(2):567-80. doi: 10.1093/toxsci/kfv206. Epub 2015 Sep 16. Toxicol Sci. 2015. PMID: 26377647 Free PMC article.
-
Lipidomic alterations in human saliva from cystic fibrosis patients.Sci Rep. 2023 Jan 12;13(1):600. doi: 10.1038/s41598-022-24429-6. Sci Rep. 2023. PMID: 36635275 Free PMC article.
References
-
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. - PubMed
-
- Bhura-Bandali FN, Suh M, Man SF, Clandinin MT. The deltaF508 mutation in the cystic fibrosis transmembrane conductance regulator alters control of essential fatty acid utilization in epithelial cells. J Nutr. 2000;130:2870–2875. - PubMed
-
- Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

