A novel synthetic analog of 5, 8-disubstituted quinazolines blocks mitosis and induces apoptosis of tumor cells by inhibiting microtubule polymerization
- PMID: 20463925
- PMCID: PMC2864768
- DOI: 10.1371/journal.pone.0010499
A novel synthetic analog of 5, 8-disubstituted quinazolines blocks mitosis and induces apoptosis of tumor cells by inhibiting microtubule polymerization
Abstract
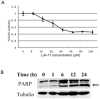
Many mitosis inhibitors are powerful anticancer drugs. Tremendous efforts have been made to identify new anti-mitosis compounds for developing more effective and less toxic anti-cancer drugs. We have identified LJK-11, a synthetic analog of 5, 8-disubstituted quinazolines, as a novel mitotic blocker. LJK-11 inhibited growth and induced apoptosis of many different types of tumor cells. It prevented mitotic spindle formation and arrested cells at early phase of mitosis. Detailed in vitro analysis demonstrated that LJK-11 inhibited microtubule polymerization. In addition, LJK-11 had synergistic effect with another microtubule inhibitor colchicine on blocking mitosis, but not with vinblastine or nocodazole. Therefore, LJK-11 represents a novel anti-microtubule structure. Understanding the function and mechanism of LJK-11 will help us to better understand the action of anti-microtubule agents and to design better anti-cancer drugs.
Conflict of interest statement
Figures









Similar articles
-
NMK-BH2, a novel microtubule-depolymerising bis (indolyl)-hydrazide-hydrazone, induces apoptotic and autophagic cell death in cervical cancer cells by binding to tubulin at colchicine - site.Biochim Biophys Acta Mol Cell Res. 2020 Oct;1867(10):118762. doi: 10.1016/j.bbamcr.2020.118762. Epub 2020 Jun 2. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32502617
-
MT7, a novel compound from a combinatorial library, arrests mitosis via inhibiting the polymerization of microtubules.Invest New Drugs. 2010 Dec;28(6):715-28. doi: 10.1007/s10637-009-9303-z. Epub 2009 Aug 25. Invest New Drugs. 2010. PMID: 19705064
-
Selective Inhibition of Spindle Microtubules by a Tubulin-Binding Quinazoline Derivative.Mol Pharmacol. 2019 Nov;96(5):609-618. doi: 10.1124/mol.119.116624. Epub 2019 Aug 30. Mol Pharmacol. 2019. PMID: 31471455
-
Microtubules and Cell Division: Potential Pharmacological Targets in Cancer Therapy.Curr Drug Targets. 2023;24(11):889-918. doi: 10.2174/1389450124666230731094837. Curr Drug Targets. 2023. PMID: 37519203 Review.
-
[Inhibitors of microtubule polymerization- new natural compounds as potential anti-cancer drugs].Postepy Hig Med Dosw (Online). 2015 May 4;69:571-85. doi: 10.5604/17322693.1151293. Postepy Hig Med Dosw (Online). 2015. PMID: 25983296 Review. Polish.
Cited by
-
NMK-TD-100, a novel microtubule modulating agent, blocks mitosis and induces apoptosis in HeLa cells by binding to tubulin.PLoS One. 2013 Oct 7;8(10):e76286. doi: 10.1371/journal.pone.0076286. eCollection 2013. PLoS One. 2013. PMID: 24116100 Free PMC article.
-
The colchicine derivative CT20126 shows a novel microtubule-modulating activity with apoptosis.Exp Mol Med. 2013 Apr 19;45(4):e19. doi: 10.1038/emm.2013.38. Exp Mol Med. 2013. PMID: 23598593 Free PMC article.
References
-
- Margolis RL, Wilson L. Microtubule treadmilling: what goes around comes around. Bioessays. 1998;20:830–836. - PubMed
-
- Kovacs P, Csaba G. Effect of drugs affecting microtubular assembly on microtubules, phospholipid synthesis and physiological indices (signalling, growth, motility and phagocytosis) in Tetrahymena pyriformis. Cell Biochem Funct. 2006;24:419–429. - PubMed
-
- Lee EA, Keutmann MK, Dowling ML, Harris E, Chan G, et al. Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol Cancer Ther. 2004;3:661–669. - PubMed
-
- Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth JA, et al. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol Cancer Ther. 2002;1:1201–1209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

