REX-1 expression and p38 MAPK activation status can determine proliferation/differentiation fates in human mesenchymal stem cells
- PMID: 20463961
- PMCID: PMC2864743
- DOI: 10.1371/journal.pone.0010493
REX-1 expression and p38 MAPK activation status can determine proliferation/differentiation fates in human mesenchymal stem cells
Abstract
Background: REX1/ZFP42 is a well-known embryonic stem cell (ESC) marker. However, the role of REX1, itself, is relatively unknown because the function of REX1 has only been reported in the differentiation of ESCs via STAT signaling pathways. Human mesenchymal stem cells (hMSCs) isolated from young tissues and cancer cells express REX1.
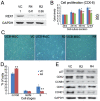
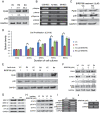
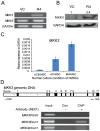
Methodology/principal finding: Human umbilical cord blood-derived MSCs (hUCB-MSCs) and adipose tissue-derived MSCs (hAD-MSCs) strongly express REX1 and have a lower activation status of p38 MAPK, but bone marrow-derived MSCs (hBM-MSCs) have weak REX1 expression and higher activation of p38 MAPK. These results indicated that REX1 expression in hMSCs was positively correlated with proliferation rates but inversely correlated with the phosphorylation of p38 MAPK. In hUCB-MSCs, the roles of REX1 and p38 MAPK were investigated, and a knockdown study was performed using a lentiviral vector-based small hairpin RNA (shRNA). After REX1 knockdown, decreased cell proliferation was observed. In REX1 knocked-down hUCB-MSCs, the osteogenic differentiation ability deteriorated, but the adipogenic potential increased or was similar to that observed in the controls. The phosphorylation of p38 MAPK in hUCB-MSCs significantly increased after REX1 knockdown. After p38 MAPK inhibitor treatment, the cell growth in REX1 knocked-down hUCB-MSCs almost recovered, and the suppressed expression levels of CDK2 and CCND1 were also restored. The expression of MKK3, an upstream regulator of p38 MAPK, significantly increased in REX1 knocked-down hUCB-MSCs. The direct binding of REX1 to the MKK3 gene was confirmed by a chromatin immunoprecipitation (ChIP) assay.
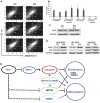
Conclusions/significance: These findings showed that REX1 regulates the proliferation/differentiation of hMSCs through the suppression of p38 MAPK signaling via the direct suppression of MKK3. Therefore, p38 MAPK and REX-1 status can determine the cell fate of adult stem cells (ASCs). These results were the first to show the role of REX1 in the proliferation/differentiation of ASCs.
Conflict of interest statement
Figures


Similar articles
-
Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways.Biochem Biophys Res Commun. 2010 Jul 16;398(1):105-10. doi: 10.1016/j.bbrc.2010.06.043. Epub 2010 Jun 15. Biochem Biophys Res Commun. 2010. PMID: 20558135
-
Mesenchymal stem cells with p38 mitogen-activated protein kinase interference ameliorate mouse ischemic stroke.Exp Biol Med (Maywood). 2023 Dec;248(23):2481-2491. doi: 10.1177/15353702231220663. Epub 2023 Dec 30. Exp Biol Med (Maywood). 2023. PMID: 38158804 Free PMC article.
-
Effect of TAK1 on osteogenic differentiation of mesenchymal stem cells by regulating BMP-2 via Wnt/β-catenin and MAPK pathway.Organogenesis. 2018 Jan 2;14(1):36-45. doi: 10.1080/15476278.2018.1455010. Organogenesis. 2018. PMID: 29913119 Free PMC article.
-
The many faces of p38 mitogen-activated protein kinase in progenitor/stem cell differentiation.Biochem J. 2012 Jul 1;445(1):1-10. doi: 10.1042/BJ20120401. Biochem J. 2012. PMID: 22702973 Review.
-
Role of p38 MAPK Signalling in Testis Development and Male Fertility.Oxid Med Cell Longev. 2022 Aug 31;2022:6891897. doi: 10.1155/2022/6891897. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36092154 Free PMC article. Review.
Cited by
-
Exposure to Insecticides Modifies Gene Expression and DNA Methylation in Hematopoietic Tissues In Vitro.Int J Mol Sci. 2023 Mar 26;24(7):6259. doi: 10.3390/ijms24076259. Int J Mol Sci. 2023. PMID: 37047231 Free PMC article.
-
Potential tumor suppressor NESG1 as an unfavorable prognosis factor in nasopharyngeal carcinoma.PLoS One. 2011;6(11):e27887. doi: 10.1371/journal.pone.0027887. Epub 2011 Nov 28. PLoS One. 2011. PMID: 22140479 Free PMC article.
-
Cancer Angiogenesis and Opportunity of Influence on Tumor by Changing Vascularization.J Pers Med. 2022 Feb 22;12(3):327. doi: 10.3390/jpm12030327. J Pers Med. 2022. PMID: 35330327 Free PMC article. Review.
-
3D Culture of MSCs on a Gelatin Microsphere in a Dynamic Culture System Enhances Chondrogenesis.Int J Mol Sci. 2020 Apr 13;21(8):2688. doi: 10.3390/ijms21082688. Int J Mol Sci. 2020. PMID: 32294921 Free PMC article.
-
The Role of Cellular Proliferation in Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells.Stem Cells Dev. 2017 Nov 1;26(21):1578-1595. doi: 10.1089/scd.2017.0071. Epub 2017 Oct 4. Stem Cells Dev. 2017. PMID: 28874101 Free PMC article.
References
-
- Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

