Novel approaches to detect serum biomarkers for clinical response to interferon-beta treatment in multiple sclerosis
- PMID: 20463963
- PMCID: PMC2864746
- DOI: 10.1371/journal.pone.0010484
Novel approaches to detect serum biomarkers for clinical response to interferon-beta treatment in multiple sclerosis
Abstract
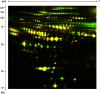
Interferon beta (IFNbeta) is the most common immunomodulatory treatment for relapsing-remitting multiple sclerosis (RRMS). However, some patients fail to respond to treatment. In this study, we identified putative clinical response markers in the serum and plasma of people with multiple sclerosis (MS) treated with IFNbeta. In a discovery-driven approach, we use 2D-difference gel electrophoresis (DIGE) to identify putative clinical response markers and apply power calculations to identify the sample size required to further validate those markers. In the process we have optimized a DIGE protocol for plasma to obtain cost effective and high resolution gels for effective spot comparison. APOA1, A2M, and FIBB were identified as putative clinical response markers. Power calculations showed that the current DIGE experiment requires a minimum of 10 samples from each group to be confident of 1.5 fold difference at the p<0.05 significance level. In a complementary targeted approach, Cytometric Beadarray (CBA) analysis showed no significant difference in the serum concentration of IL-6, IL-8, MIG, Eotaxin, IP-10, MCP-1, and MIP-1alpha, between clinical responders and non-responders, despite the association of these proteins with IFNbeta treatment in MS.
Conflict of interest statement
Figures



References
-
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39:285–294. - PubMed
-
- Kappos L, Weinshenker B, Pozzilli C, Thompson AJ, Dahlke F, et al. Interferon beta-1b in secondary progressive MS: a combined analysis of the two trials. Neurology. 2004;63:1779–1787. - PubMed
-
- Wandinger KP, Lunemann JD, Wengert O, Bellman-Strobl J, Aktas O, et al. TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet. 2003;361:2036–2043. - PubMed
-
- Durelli L, Oggero A, Verdun E, Barbero P, Pipieri A, et al. Interferon-beta dose and efficacy: the OPTIMS study. Neurol Sci. 2001;22:201–203. - PubMed
-
- Sorensen PS, Ross C, Clemmesen KM, Bendtzen K, Frederikson JL, et al. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. The Lancet. 2003;362:1184–1191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

