Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica
- PMID: 20463974
- PMCID: PMC2864757
- DOI: 10.1371/journal.pone.0010455
Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica
Abstract
Background: Neuromyelitis optica (NMO), a severe demyelinating disease, represents itself with optic neuritis and longitudinally extensive transverse myelitis. Serum NMO-IgG autoantibodies (Abs), a specific finding in NMO patients, target the water channel protein aquaporin-4 (AQP4), which is expressed as a long (M-1) or a short (M-23) isoform.
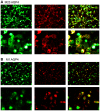
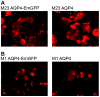
Methodology/principal findings: The aim of this study was to analyze serum samples from patients with NMO and controls for the presence and epitope specificity of IgG and IgM anti-AQP4 Abs using an immunofluorescence assay with HEK293 cells expressing M-1 or M-23 human AQP4. We included 56 patients with definite NMO (n = 30) and high risk NMO (n = 26), 101 patients with multiple sclerosis, 27 patients with clinically isolated syndromes (CIS), 30 patients with systemic lupus erythematosus (SLE) or Sjögren's syndrome, 29 patients with other neurological diseases and 47 healthy controls. Serum anti-AQP4 M-23 IgG Abs were specifically detected in 29 NMO patients, 17 patients with high risk NMO and two patients with myelitis due to demyelination (CIS) and SLE. In contrast, IgM anti-AQP4 Abs were not only found in some NMO and high risk patients, but also in controls. The sensitivity of the M-23 AQP4 IgG assay was 97% for NMO and 65% for high risk NMO, with a specificity of 100% compared to the controls. Sensitivity with M-1 AQP4 transfected cells was lower for NMO (70%) and high risk NMO (39%). The conformational epitopes of M-23 AQP4 are the primary targets of NMO-IgG Abs, whereas M-1 AQP4 Abs are developed with increasing disease duration and number of relapses.
Conclusions: Our results confirm M-23 AQP4-IgG Abs as reliable biomarkers in patients with NMO and high risk syndromes. M-1 and M-23 AQP4-IgG Abs are significantly associated with a higher number of relapses and longer disease duration.
Conflict of interest statement
Figures

Similar articles
-
Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders.J Neuroinflammation. 2011 Dec 28;8:184. doi: 10.1186/1742-2094-8-184. J Neuroinflammation. 2011. PMID: 22204662 Free PMC article.
-
Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica.Tohoku J Exp Med. 2006 Dec;210(4):307-13. doi: 10.1620/tjem.210.307. Tohoku J Exp Med. 2006. PMID: 17146196
-
Change in autoantibody and cytokine responses during the evolution of neuromyelitis optica in patients with systemic lupus erythematosus: A preliminary study.Mult Scler. 2016 Aug;22(9):1192-201. doi: 10.1177/1352458515613165. Epub 2015 Oct 29. Mult Scler. 2016. PMID: 26514978
-
Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature.Brain Pathol. 2013 Nov;23(6):661-83. doi: 10.1111/bpa.12084. Brain Pathol. 2013. PMID: 24118483 Free PMC article. Review.
-
Neuromyelitis optica pathogenesis and aquaporin 4.J Neuroinflammation. 2008 May 29;5:22. doi: 10.1186/1742-2094-5-22. J Neuroinflammation. 2008. PMID: 18510734 Free PMC article. Review.
Cited by
-
Rare variants and HLA haplotypes associated in patients with neuromyelitis optica spectrum disorders.Front Immunol. 2022 Oct 4;13:900605. doi: 10.3389/fimmu.2022.900605. eCollection 2022. Front Immunol. 2022. PMID: 36268024 Free PMC article.
-
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin.J Neuroinflammation. 2016 Sep 26;13(1):279. doi: 10.1186/s12974-016-0717-1. J Neuroinflammation. 2016. PMID: 27788675 Free PMC article.
-
Distinct serum and cerebrospinal fluid cytokine and chemokine profiles in autoantibody-associated demyelinating diseases.Mult Scler J Exp Transl Clin. 2019 May 15;5(2):2055217319848463. doi: 10.1177/2055217319848463. eCollection 2019 Apr-Jun. Mult Scler J Exp Transl Clin. 2019. PMID: 31205739 Free PMC article.
-
Aquaporin-4 antibody testing: direct comparison of M1-AQP4-DNA-transfected cells with leaky scanning versus M23-AQP4-DNA-transfected cells as antigenic substrate.J Neuroinflammation. 2014 Jul 29;11:129. doi: 10.1186/1742-2094-11-129. J Neuroinflammation. 2014. PMID: 25074611 Free PMC article.
-
Epidemiology of Pediatric NMOSD in Germany and Austria.Front Neurol. 2020 May 15;11:415. doi: 10.3389/fneur.2020.00415. eCollection 2020. Front Neurol. 2020. PMID: 32670175 Free PMC article.
References
-
- Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999;53:1107–1114. - PubMed
-
- Cree B. Neuromyelitis optica: diagnosis, pathogenesis, and treatment. Curr Neurol Neurosci Rep. 2008;8:427–433. - PubMed
-
- Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology. 2003;60:848–853. - PubMed
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. - PubMed
-
- Jarius S, Franciotta D, Bergamaschi R, Wright H, Littleton E, et al. NMO-IgG in the diagnosis of neuromyelitis optica. Neurology. 2007;68:1076–1077. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

