Progressive multifocal leukoencephalopathy and promyelocytic leukemia nuclear bodies: a review of clinical, neuropathological, and virological aspects of JC virus-induced demyelinating disease
- PMID: 20464404
- PMCID: PMC2910879
- DOI: 10.1007/s00401-010-0694-x
Progressive multifocal leukoencephalopathy and promyelocytic leukemia nuclear bodies: a review of clinical, neuropathological, and virological aspects of JC virus-induced demyelinating disease
Abstract
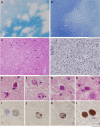
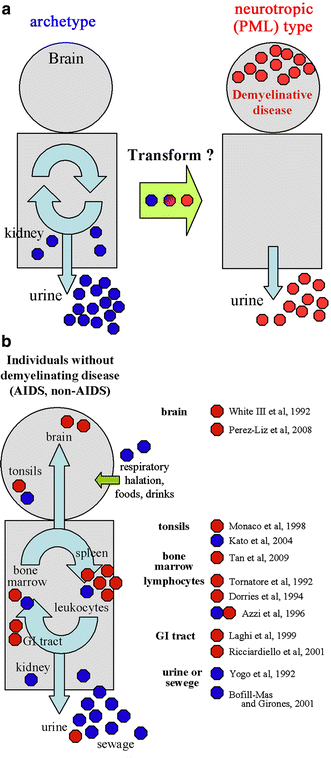
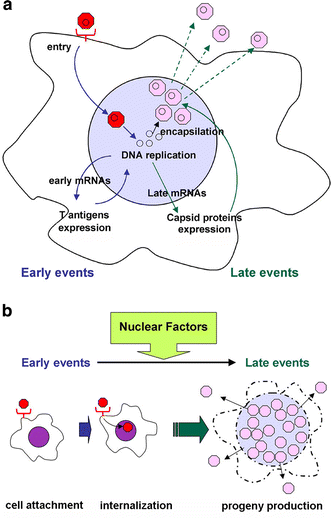
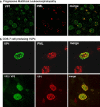
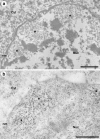
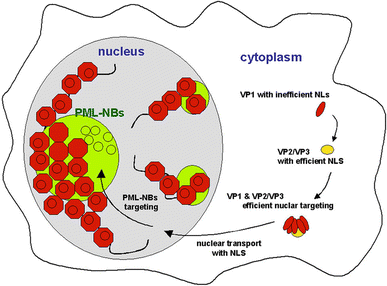
Progressive multifocal leukoencephalopathy is a fatal viral-induced demyelinating disease that was once rare but has become more prevalent today. Over the past decades, much has been learned about the disease from molecular study of the etiological agent of the disease, JC virus. Recently, promyelocytic leukemia nuclear bodies (PML-NBs), punctuate structures for important nuclear functions in eukaryotic cells, were identified as an intranuclear target of JC virus infection. Neuropathologically, JC virus-infected glial cells display diffuse amphophilic viral inclusions by hematoxylin-eosin staining (full inclusions), a diagnostic hallmark of this disease. Recent results using immunohistochemistry, however, revealed the presence of punctate viral inclusions preferentially located along the inner nuclear periphery (dot-shaped inclusions). Dot-shaped inclusions reflect the accumulation of viral progeny at PML-NBs, which may be disrupted after viral replication. Structural changes to PML-NBs have been reported for a variety of human diseases, including cancers and neurodegenerative disorders. Thus, PML-NBs may provide clues to the further pathogenesis of JC virus-induced demyelinating disease. Here, we review what we have learned since the disease entity establishment, including a look at recent progress in understanding the relationship between JC virus, etiology and PML-NBs.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

