Metabolic acetate therapy improves phenotype in the tremor rat model of Canavan disease
- PMID: 20464498
- PMCID: PMC2877317
- DOI: 10.1007/s10545-010-9100-z
Metabolic acetate therapy improves phenotype in the tremor rat model of Canavan disease
Abstract
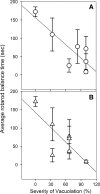
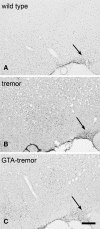
Genetic mutations that severely diminish the activity of aspartoacylase (ASPA) result in the fatal brain dysmyelinating disorder, Canavan disease. There is no effective treatment. ASPA produces free acetate from the concentrated brain metabolite, N-acetylaspartate (NAA). Because acetyl coenzyme A is a key building block for lipid synthesis, we postulated that the inability to catabolize NAA leads to a brain acetate deficiency during a critical period of CNS development, impairing myelination and possibly other aspects of brain development. We tested the hypothesis that acetate supplementation during postnatal myelination would ameliorate the severe phenotype associated with ASPA deficiency using the tremor rat model of Canavan disease. Glyceryltriacetate (GTA) was administered orally to tremor rats starting 7 days after birth, and was continued in food and water after weaning. Motor function, myelin lipids, and brain vacuolation were analyzed in GTA-treated and untreated tremor rats. Significant improvements were observed in motor performance and myelin galactocerebroside content in tremor rats treated with GTA. Further, brain vacuolation was modestly reduced, and these reductions were positively correlated with improved motor performance. We also examined the expression of the acetyl coenzyme A synthesizing enzyme acetyl coenzyme A synthase 1 and found upregulation of expression in tremor rats, with a return to near normal expression levels in GTA-treated tremor rats. These results confirm the critical role played by NAA-derived acetate in brain myelination and development, and demonstrate the potential usefulness of acetate therapy for the treatment of Canavan disease.
Figures






References
-
- Bailey JW, Barker RL, Karlstad MD. Total parenteral nutrition with short- and long-chain triglycerides: triacetin improves nitrogen balance in rats. J Nutr. 1992;122(9):1823–1829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

