Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children
- PMID: 20464739
- PMCID: PMC4169792
- DOI: 10.1002/14651858.CD005535.pub2
Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children
Abstract
Background: Long-acting inhaled ss(2)-adrenergic agonists (LABAs) are recommended as 'add-on' medication to inhaled corticosteroids (ICS) in the maintenance therapy of asthmatic adults and children aged two years and above.
Objectives: To quantify in asthmatic patients the safety and efficacy of the addition of LABAs to ICS in patients insufficiently controlled on ICS alone.
Search strategy: We identified randomised controlled trials (RCTs) through electronic database searches (the Cochrane Airways Group Specialised Register, MEDLINE, EMBASE and CINAHL), bibliographies of RCTs and correspondence with manufacturers until May 2008.
Selection criteria: We included RCTs if they compared the addition of inhaled LABAs versus placebo to the same dose of ICS in children aged two years and above and in adults.
Data collection and analysis: Two review authors independently assessed studies for methodological quality and extracted data. We obtained confirmation from the trialists when possible. The primary endpoint was the relative risk (RR) of asthma exacerbations requiring rescue oral corticosteroids. Secondary endpoints included pulmonary function tests (PFTs), rescue beta2-agonist use, symptoms, withdrawals and adverse events.
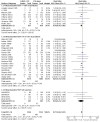
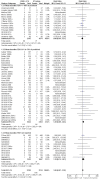
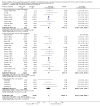
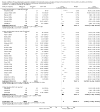
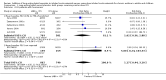
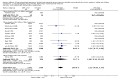
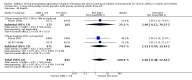
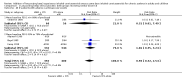
Main results: Seventy-seven studies met the entry criteria and randomised 21,248 participants (4625 children and 16,623 adults). Participants were generally symptomatic at baseline with moderate airway obstruction despite their current ICS regimen. Formoterol or salmeterol were most frequently added to low-dose ICS (200 to 400 microg/day of beclomethasone (BDP) or equivalent) in 49% of the studies. The addition of a daily LABA to ICS reduced the risk of exacerbations requiring oral steroids by 23% from 15% to 11% (RR 0.77, 95% CI 0.68 to 0.87, 28 studies, 6808 participants). The number needed to treat with the addition of LABA to prevent one use of rescue oral corticosteroids is 41 (29, 72), although the event rates in the ICS groups varied between 0% and 38%. Studies recruiting adults dominated the analysis (6203 adult participants versus 605 children). The subgroup estimate for paediatric studies was not statistically significant (RR 0.89, 95% CI 0.58 to 1.39) and includes the possibility of the superiority of ICS alone in children.Higher than usual dose of LABA was associated with significantly less benefit. The difference in the relative risk of serious adverse events with LABA was not statistically significant from that of ICS alone (RR 1.06, 95% CI 0.87 to 1.30). The addition of LABA led to a significantly greater improvement in FEV(1) (0.11 litres, 95% 0.09 to 0.13) and in the proportion of symptom-free days (11.88%, 95% CI 8.25 to 15.50) compared to ICS monotherapy. It was also associated with a reduction in the use of rescue short-acting ss(2)-agonists (-0.58 puffs/day, 95% CI -0.80 to -0.35), fewer withdrawals due to poor asthma control (RR 0.50, 95% CI 0.41 to 0.61), and fewer withdrawals due to any reason (RR 0.80, 95% CI 0.75 to 0.87). There was no statistically significant group difference in the risk of overall adverse effects (RR 1.00, 95% 0.97 to 1.04), withdrawals due to adverse health events (RR 1.04, 95% CI 0.86 to 1.26) or any of the specific adverse health events.
Authors' conclusions: In adults who are symptomatic on low to high doses of ICS monotherapy, the addition of a LABA at licensed doses reduces the rate of exacerbations requiring oral steroids, improves lung function and symptoms and modestly decreases use of rescue short-acting ss(2)-agonists. In children, the effects of this treatment option are much more uncertain. The absence of group difference in serious adverse health events and withdrawal rates in both groups provides some indirect evidence of the safety of LABAs at usual doses as add-on therapy to ICS in adults, although the width of the confidence interval precludes total reassurance.
Conflict of interest statement
In the past five years, Francine Ducharme received some research funding from GSK and Merck & Co, USA and gave CME conferences supported by Merck Frosst. M Ni Chroinin, IR Greenstone, A Danish, H Magalinos, V Masse, M Julien and T Lasserson report no conflict of interest.
Figures








































































Update of
-
Long-acting beta2-agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD005535. doi: 10.1002/14651858.CD005535. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2010 May 12;(5):CD005535. doi: 10.1002/14651858.CD005535.pub2. PMID: 16235410 Updated.
References
References to studies included in this review
Akpinarli 1999 {published data only}
Aubier 1999a {published data only}
-
- Aubier M, Pieters WR, Schlosser NJ, Steinmetz KO. Salmeterol/fluticasone propionate (50/500 mug) in combination in a Diskus(TM) inhaler (Seretide(TM)) is effective and safe in the treatment of steroid‐dependent asthma. Respiratory Medicine 1999;93(12):876‐84. - PubMed
-
- Pieters W, Ringdal N, Aubier M, Chapman KR, Huskisson SC. A new inhaler combination containing salmeterol and fluticasone propionate is well‐tolerated in long‐term use. European Respiratory Journal. 1998; Vol. 12, issue Suppl 29:P164.
-
- Pieters WR, Lundback B, Sondhi S, Price MJ. Cost effectiveness analysis of salmeterol/fluticasone propionate 50/500mcg vs fluticasone propionate 500mcg in patients with corticosteroid‐dependent asthma. Pharmacoeconomics 1999;16(Suppl 2):29‐34.
-
- Pieters WR, Sondhi S, Price MJ, Thwaites RM, Nyth A. The cost effectiveness of salmeterol/fluticasone propionate 50/500 microgram combination inhaler versus fluticasone propionate 500 microgram in patients with chronic asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:P2458.
-
- Pieters WR, Steinmetz KO, Aubier M, Johnson L, Gomez E, Bogolubov M. Effectiveness of a new salmeterol/fluticasone propionate (50/500µg) combination inhaler in patients with reversible airways obstruction. European Respiratory Journal 1998; Vol. 12, issue Suppl 28:35s.
Aubier 1999b {published data only}
-
- Aubier M, Pieters WR, Schlosser NJ, Steinmetz KO. Salmeterol/fluticasone propionate (50/500 mug) in combination in a Diskus(TM) inhaler (Seretide(TM)) is effective and safe in the treatment of steroid‐dependent asthma. Respiratory Medicine 1999;93(12):876‐84. - PubMed
-
- Pieters W, Ringdal N, Aubier M, Chapman KR, Huskisson SC. A new inhaler combination containing salmeterol and fluticasone propionate is well‐tolerated in long‐term use. European Respiratory Journal. 1998; Vol. 12, issue Suppl 29:P164.
-
- Pieters WR, Lundback B, Sondhi S, Price MJ. Cost effectiveness analysis of salmeterol/fluticasone propionate 50/500mcg vs fluticasone propionate 500mcg in patients with corticosteroid‐dependent asthma. Pharmacoeconomics 1999;16(Suppl 2):29‐34.
-
- Pieters WR, Sondhi S, Price MJ, Thwaites RM, Nyth A. The cost effectiveness of salmeterol/fluticasone propionate 50/500 microgram combination inhaler versus fluticasone propionate 500 microgram in patients with chronic asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:P2458.
-
- Pieters WR, Steinmetz KO, Aubier M, Johnson L, Gomez E, Bogolubov M. Effectiveness of a new salmeterol/fluticasone propionate (50/500µg) combination inhaler in patients with reversible airways obstruction. European Respiratory Journal 1998; Vol. 12, issue Suppl 28:35s.
Bailey 2008 {unpublished data only}
-
- Bailey W, Castro M, Matz J, White M, Dransfield M, Yancey S, et al. Asthma exacerbations in African Americans treated for 1 year with combination fluticasone propionate and salmeterol or fluticasone propionate alone. Current Medical Research and Opinion 2008;24(6):1669‐82. - PubMed
-
- Glaxo Smith Kline (SFA103153). A multicenter, randomized, double‐blind, parallel group, 52‐week comparison of asthma control and measures of airway inflammation in subjects of African descent receiving fluticasone propionate/salmeterol 100/50mcg DISKUS™ BID or fluticasone propionate 100mcg DISKUS™ BID alone. http://www.gsk.ctr.co.uk 2007 (accessed 30 April 2008).
Boyd 1995 {published data only}
-
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. European Respiratory Journal 1995;8:1494‐8. - PubMed
Buhl 2003a {published data only}
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Improved and maintained asthma control with once‐daily budesonide/formoterol single inhaler in mild‐to‐moderate persistent asthma. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:21s.
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Once daily Symbicort (budesonide/eformoterol in a single inhaler) is effective in moderate‐persistent asthma. Thorax 2001;56(Suppl 3):iii62.
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Once‐daily budesonide/formoterol via a single inhaler is effective in mild‐to‐moderate persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:21s.
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA, Naya IP, Eksstrom T. Once daily budesonide /formoterol in a single inhaler in adults with moderate persistent asthma. Respiratory Medicine 2003;97(4):323‐30. - PubMed
-
- Buhl R, Zetterstrom O, Mellem H, Perpina M, Hedman J, O'Neill S, et al. Improved asthma control with budesonide/formoterol via a single inhaler compared with budesonide alone, in moderate persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:48s. - PubMed
Buhl 2003b {published data only}
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Improved and maintained asthma control with once‐daily budesonide/formoterol single inhaler in mild‐to‐moderate persistent asthma. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:21s.
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Once daily Symbicort (budesonide/eformoterol in a single inhaler) is effective in moderate‐persistent asthma. Thorax 2001;56(Suppl 3):iii 62.
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Once‐daily budesonide/formoterol via a single inhaler is effective in mild‐to‐moderate persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:21s.
-
- Buhl R, Creemers JPHM, Vondra V, Martelli NA, Naya IP, Eksstrom T. Once daily budesonide /formoterol in a single inhaler in adults with moderate persistent asthma. Respiratory Medicine 2003;97:323‐30. - PubMed
-
- Buhl R, Zetterstrom O, Mellem H, Perpina M, Hedman J, O'Neill S, et al. Improved asthma control with budesonide/formoterol via a single inhaler compared with budesonide alone, in moderate persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:48s. - PubMed
D'Urzo 2001 {published data only}
-
- D'Urzo AD, Chapman KR, Cartier A, Hargreave FE, Fitzgeerald M, Tesarowski D. Effectiveness and safety of salmeterol in non‐specialist practice settings. Chest 2001;119:714‐9. - PubMed
-
- SLGQ94 (521/180) (GSK). A multicenter, randomized, double‐blind, parallel‐group trial to evaluate the long‐term efficacy and safety of inhaled salmeterol 50μg BID compared to short‐acting β2‐agonists as‐needed in adult patients with asthma. http://ctr.gsk.co.uk 2007 (accessed 20 June 2008).
D5896C0001a {unpublished data only}
-
- AstraZeneca (D5896C00001). A randomized, double‐blind, active‐controlled, parallel‐group, single‐dummy, multicenter, 12 week study to assess the efficacy and safety of SYMBICORT® pMDI 160/4.5 μg x 2 actuations once‐daily (qd) compared to SYMBICORT pMDI 80/4.5 μg x 2 actuations qd, SYMBICORT pMDI80/4.5 μg x 2 actuations twice‐daily (bid) and to budesonide pMDI 160 μgx 2 actuations qd in asthmatic subjects 12 years of age and older. http://www.astrazenecaclinicaltrials.com 2005 (accessed 30 April 2008).
D5896C0001b {unpublished data only}
-
- AstraZeneca (D5896C00001). A randomized, double‐blind, active‐controlled, parallel‐group, single‐dummy, multicenter, 12 week study to assess the efficacy and safety of SYMBICORT® pMDI 160/4.5 μg x 2 actuations once‐daily (qd) compared to SYMBICORT pMDI 80/4.5 μg x 2 actuations qd, SYMBICORT pMDI80/4.5 μg x 2 actuations twice‐daily (bid) and to budesonide pMDI 160 μgx 2 actuations qd in asthmatic subjects 12 years of age and older. http://www.astrazenecaclinicaltrials.com 2005 (accessed 30 April 2008).
Fitzgerald 1999 {published data only}
-
- FitzGerald JM, Chapman KR, Della Cioppa G, Stubbing D, Fairbarn MS, Till MD, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. The Canadian FO/OD1 Study Group. Journal of Allergy & Clinical Immunology 1999;103(3 pt 1):427‐35. - PubMed
Gardiner 1994 {published data only}
-
- Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH. Bronchoalveolar lavage inflammatory indices in asthmatics. American Journal of Respiratory & Critical Care Medicine 1994;150(4):1006‐11. - PubMed
GOAL {published and unpublished data}
-
- Anonymous. GSK asthma trial suggests total control is possible. Pharmaceutical Journal 2004;273(7322):594.
-
- Arthurs R. Gaining optimal asthma control. Practice Nurse 2004;Suppl:3‐8.
-
- Bateman E, Boushey H, Bousquet J, Busse W, Clark T, Pauwels R, et al. Achievement for guideline based asthma control with salmeterol/fluticasone propionate compared with fluticasone propionate alone; results of Goal study. Triennial World Asthma Meeting, Thailand (16‐19 February). 2004.
-
- Bateman E, Boushey H, Bousquet J, Busse W, Clark T, Pauwels R, et al. Achieving and maintaining guideline defined asthma control with salmeterol/fluticasone propionate versus fluticasone propionate alone: the results of the GOAL study. American Journal of Respiratory & Critical Care Medicine 2004;169(7):A87. - PubMed
-
- Bateman E, Pauwels R, Boushey H, Bousquet J, Busse W, Clark T, et al. Aiming for total control of asthma significantly improves asthma‐related quality of life: salmeterol/fluticasone propionate versus fluticasone propionate alone. American Journal of Respiratory & Critical Care Medicine 2004;169(7):A87.
Green 2006 {published data only}
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Neale N, Parker D, et al. Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. European Respiratory Journal 2006;27(6):1144‐51. - PubMed
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Pavord ID. A placebo controlled comparison of formoterol, montelukast or higher dose of inhaled budesonide in subjects with symptomatic asthma despite treatment with low dose inhaled budesonide. American Thoracic Society 99th International Conference. 2003:B036 Poster H82.
Houghton 2007 {published data only}
Hultquist 2000 {unpublished data only}
-
- AstraZeneca (SD 004 0216). Oxis turbuhaler® (formoterol), Accolate® (zafirlukast) or placebo as add on treatment to Pulmicort Turbuhaler® (budesonide) in asthmatic patients on inhaled steroids. http://www.astrazenecaclinicaltrials.com 2000 (accessed 30 April 2008).
-
- Hultquist C. Unpublished data. Personal communication.
Ind 2003 {published data only}
-
- Ind P, Haughney J, Price D, Rosen J‐P, Kennelly J. Four months adjustable or fixed BD dosing with budesonide /Formoterol in a single inhaler reduces symptom severity. Thorax 2002; Vol. 57, issue Suppl 3:iii88.
-
- Ind PW, Dal Negro R, Colman N, Fletcher CP, Browning DC, James MH. Inhaled fluticasone propionate and salmeterol in moderate adult asthma I: lung function and symptoms. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A416.
-
- Ind PW, Dal Negro R, Colman N, Fletcher CP, Browning DC, James MH. Inhaled fluticasone propionate and salmeterol in moderate adult asthma II: exacerbations. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A415.
-
- Ind PW, Dal Negro R, Colman NC, Fletcher CP, Browning D, James MH. Addition of salmeterol to fluticasone propionate treatment in moderate to severe asthma. Respiratory Medicine 2003;97(5):555‐62. - PubMed
-
- SLGQ97. A multi‐centre double‐blind, parallel group study to evaluate the relative clinical benefits of three treatment interventions: i) salmeterol xinafoate 50 mcg bd plus fluticasone propionate 250 mcg bd; ii) fluticasone propionate 500 mcg bd; iii) fluticasone propionate 250 mcg bd, in adult asthmatic subjects poorly controlled on current inhaled corticosteroids.. http://ctr.gsk.co.uk 2005.
Jenkins 2006a {published and unpublished data}
-
- Jenkins C, Kolarikova R, Kuna P, Caillaud D, Sanchis J, Popp W, et al. Efficacy and safety of high‐dose budesonide/formoterol (Symbicort(R)) compared with budesonide administered either concomitantly with formoterol or alone in patients with persistent symptomatic asthma. Respirology 2006;11(3):276‐86. - PubMed
-
- SD 039 0689. Efficacy and safety of Symbicort® (budesonide/ formoterol) 1280/36 mcg daily delivered dose compared to Pulmicort® (budesonide) 1600 mcg metered dose and Pulmicort (budesonide) 1600 mcg metered dose plus Oxis® (formoterol) 36 mcg delivered dose all delivered via Turbuhaler® in steroid‐using asthmatic adolescents and adults. A double‐blind, double‐dummy, randomized, parallel group, phase III, multicentre study. http://www.astrazeneca.com 2005.
Jenkins 2006b {published data only}
-
- Jenkins C, Kolarikova R, Kuna P, Caillaud D, Sanchis J, Popp W, et al. Efficacy and safety of high‐dose budesonide/formoterol (Symbicort(R)) compared with budesonide administered either concomitantly with formoterol or alone in patients with persistent symptomatic asthma. Respirology 2006;11(3):276‐86. - PubMed
-
- SD 039 0689. Efficacy and safety of Symbicort® (budesonide/ formoterol) 1280/36 mcg daily delivered dose compared to Pulmicort® (budesonide) 1600 mcg metered dose and Pulmicort (budesonide) 1600 mcg metered dose plus Oxis® (formoterol) 36 mcg delivered dose all delivered via Turbuhaler® in steroid‐using asthmatic adolescents and adults. A double‐blind, double‐dummy, randomized, parallel group, phase III, multicentre study. http://www.astrazeneca.com 2005.
Kavaru 2000 {published and unpublished data}
-
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life in asthma patients. Journal of Allergy and Clinical Immunology 2001; Vol. 107, issue 2:S246.
-
- Edin HM, Prillaman B, Baitinger LA, House K, Shah TP. Improved ability to perform strenuous activities after treatment with fluticasone propionate‐salmeterol combination. American Journal of Respiratory & Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A112.
-
- Edwards T, Gross G, Mitchell D, Chervinsky P, Woodring A, Baitinger L, et al. The salmeterol xinafoate/fluticasone propionate dry powder combination product via diskus® inhaler improves asthma control compared to salmeterol xinafoate or fluticasone propionate dry powder alone. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A414.
-
- Johansson G, Price MJ, Sondhi S. Cost‐effectiveness analysis of salmeterol/fluticasone propionate 50/100mug vs fluticasone propionate 100mug in adults and adolescents with asthma III. Pharmacoeconomics 1999;16(Suppl 2):15‐21.
-
- Kavuru M, Melamed J, Gross G, Laforce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomised, double blind,placebo controlled trial. Journal of Allergy and Clinical Immunology 2000;105(6):1108‐16. - PubMed
Kemp 1998 {published data only}
-
- Kemp JP, Cook DA, Incaudo GA, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1998;101:188‐95. - PubMed
Koopmans 2006 {published data only}
-
- SAS30013. A study to compare the long term effects on airway inflammation of Seretide versus Flixotide in adult subjects with asthma. http://www.ctr.gsk.co.uk 2004.
Kuna 2006 {published data only}
-
- AstraZeneca (SD 039 0665). Symbicort low dose once daily in mild to moderate asthmatic patients. http://www.astrazenecaclinicaltrials.com 2000 (accessed 30 April 2008).
-
- Kuna P, Creemers JPHM, Vondra V, Black PN, Lindqvist A, Nihlen U, et al. Once‐daily dosing with budesonide/formoterol compared with twice‐daily budesonide/formoterol and once‐daily budesonide in adults with mild to moderate asthma. Respiratory Medicine 2006;100(12):2151‐9. - PubMed
Langton Hewer 1995 {published data only}
-
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrims progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89:435‐40. - PubMed
Leblanc 1996 {published data only}
-
- Leblanc P, Knight A, Kreisman H, Borkhoff CM, Johnston PR. A placebo‐controlled, crossover comparison of salmeterol and salbutamol in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1996;154:324‐8. - PubMed
Li 1999 {published data only}
-
- Li X, Ward C, Thien F, Bish R, Bamford T, Bao X, et al. An antiinflammatory effect of salmeterol, a long‐acting B2‐agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. American Journal of Respiratory & Critical Care Medicine 1999;160:1493‐9. - PubMed
-
- Reid DW, Ward C, Wang N, Zheng L, Bish R, Orsida B, et al. Possible anti‐inflammatory effect of salmeterol against interleukin‐8 and neutrophil activation in asthma in vivo. European Respiratory Journal 2003;21(6):994‐9. - PubMed
Malone 2005 {published and unpublished data}
-
- Dorinsky P, Emmett A, Sutton L. Reduced risk for asthma exacerbations in pediatric patients receiving salmeterol plus inhaled corticosteroids (ICS) vs ICS alone [Abstract]. European Respiratory Journal 2004;24(Suppl 48):308s.
-
- House K, Dorinsky PM, Stauffer J, Schoaf L, Ellsworth A. The safety of fluticasone propionate/salmeterol Diskus(R) in pediatric patients ages 4 ‐11 with asthma [Abstract]. Chest 2004;126(Suppl 4):911S.
-
- Malone R, LaForce C, Nimmagadda S, Schoaf L, House K, Ellsworth A, et al. The safety of twice‐daily treatment with fluticasone propionate and salmeterol in pediatric patients with persistent asthma. Annals of Allergy, Asthma, and Immunology 2005;95(1):66‐71. - PubMed
-
- SAS30031(GSK). A randomized, double‐blind, 12‐week trial evaluating the safety of the fluticasone propionate/salmeterol DISKUS combination product 100/50mcg BID versus fluticasone propionate DISKUS 100mcg BID in symptomatic pediatric subjects (4‐11 years) with asthma. http://www.ctr.gsk.co.uk 2005 (accessed 30 April 2008).
-
- Scott C, Wu W, Ellsworth A, Crim C. Efficacy and safety of fluticasone propionate/salmeterol DISKUS and fluticasone propionate DISKUS and HFA in children [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1057.
Meijer 1995 {published data only}
-
- Meijer GG, Postma DS, Mulder PG, Aalderen WM. Long‐term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 1995;152:1887‐92. - PubMed
Molimard 2001 {published data only}
-
- Molimard M, Bourcereau J, Gros V, Bourdeix I, Leynadier F, Duroux P. Comparison between formoterol 12 ug bid and on‐demand salbutamol in moderate persistent asthma. Respiratory Medicine 2001;95(1):64‐70. - PubMed
Morice 2008a {published and unpublished data}
-
- AstraZeneca. A 12‐week randomised, double‐blind, parallel‐group, multicentre phase‐III study to compare the efficacy and safety of Symbicort® pMDI (budesonide/formoterol 80/4.5 mcg 2 actuations b.i.d., delivered dose) with that of Pulmicort® pMDI (budesonide 100 mcg 2 actuations b.i.d., metered dose) and Symbicort Turbuhaler® (budesonide/formoterol 80/4.5 mcg 2 actuations b.i.d., delivered dose) in children with asthma. http://www.astrazenecaclinicaltrials.com 2007 (accessed 4 January 2008).
-
- Morice AH, Peterson S, Beckman O, Kukova Z. Efficacy and safety of a new pressurised metered‐dose inhaler formulation of budesonide/formoterol in children with asthma: A superiority and therapeutic equivalence study. Pulmonology Pharmacology Therapeutics 2008;21(1):152‐9. - PubMed
Morice 2008b {published data only}
-
- AstraZeneca. A 12‐week randomised, double‐blind, parallel‐group, multicentre phase‐III study to compare the efficacy and safety of Symbicort®pMDI (budesonide/formoterol 80/4.5 mcg 2 actuations b.i.d., delivered dose) with that of Pulmicort®pMDI (budesonide 100 mcg 2 actuations b.i.d., metered dose) and Symbicort Turbuhaler® (budesonide/formoterol 80/4.5 mcg 2 actuations b.i.d., delivered dose) in children with asthma. http://www.astrazenecaclinicaltrials.com 2007 (accessed 4 January 2008).
-
- Morice AH, Peterson S, Beckman O, Kukova Z. Efficacy and safety of a new pressurised metered‐dose inhaler formulation of budesonide/formoterol in children with asthma: a superiority and therapeutic equivalence study. Pulmonology Pharmacology Therapeutics 2008; Vol. 21, issue 1:152‐9. - PubMed
Nathan 2006 {published and unpublished data}
-
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life. Journal of Allergy & Clinical Immunology 2001;107(2):S246.
-
- Nathan RA, Mitchell D, Condemi J, Heller A, Schoaf L, Herrle M, et al. Cardiovascular and hypothalamic‐pituitary‐adrenal axis safety of fluticasone propionate/salmeterol HFA MDI in adolescent and adult patients with asthma. American Journal for Respiratory & Critical Care Medicine 2001;163(5):A863.
-
- Nathan RA, Rooklin A, Schoaf L, Scott C, Ellsworth A, House K, et al. Efficacy and tolerability of fluticasone propionate/salmeterol administered twice daily via hydrofluoroalkane 134a metered‐dose inhaler in adolescent and adult patients with persistent asthma: a randomized, double‐blind, placebo‐controlled, 12‐week study. Clinical Therapeutics 2006;28(1):73‐85. - PubMed
-
- Pearlman DS, Kent E, Lanz MJ, Peden D, Baitinger L, Herrle M, et al. Fluticasone propionate/salmeterol HFA MDI has a rapid onset of effect in asthmatics treated with short or long‐acting beta‐agonists (BA) or inhaled corticosteroids (ICS). American Journal of Respiratory & Critical Care Medicine 2001;163(5):A865.
-
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The fluticasone propionate/salmeterol HFA MDI is significantly more efficacious in treating asthma than placebo HFA MDI, fluticasone propionate CFC MDI or salmeterol CFC MDI. Journal of Allergy and Clinical Immunology 2001;107(2):100s.
Noonan 2006a {published data only}
-
- Noonan M, Rosenwasser LJ, Martin P, O'Brien CD, O'Dowd L. Efficacy and safety of budesonide and formoterol in one pressurised metered‐dose inhaler in adults and adolescents with moderate to severe asthma: a randomised clinical trial. Drugs 2006;66(17):2235‐54. - PubMed
-
- SD 039 0717 (AstraZeneca). A twelve‐week, randomized, double‐blind, double‐dummy, placebo‐controlled trial of Symbicort® (160/4.5 μg) versus its mono‐products (budesonide and formoterol) in adolescents (≥12 years of age) and adults with asthma. http://www.astrazenecaclinicaltrials.com 2004 (accessed 13 June 2008).
Noonan 2006b {published data only}
-
- Noonan M, Rosenwasser LJ, Martin P, O'Brien CD, O'Dowd L. Efficacy and safety of budesonide and formoterol in one pressurised metered‐dose inhaler in adults and adolescents with moderate to severe asthma: a randomised clinical trial. Drugs 2006;66(17):2235‐54. - PubMed
-
- SD 039 0717 (AstraZeneca). A twelve‐week, randomized, double‐blind, double‐dummy, placebo‐controlled trial of Symbicort® (160/4.5 μg) versus its mono‐products (budesonide and formoterol) in adolescents (≥12 years of age) and adults with asthma. http://www.astrazenecaclinicaltrials.com 2004 (accessed 13 June 2008).
Norhaya 1999 {published data only}
-
- Norhaya MR, Yap TM, Zainudin BMZ. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Repirology 1999;4(1):77‐81. - PubMed
O'Byrne 2001a {published data only}
-
- Barnes PJ, O'Byrne PM, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Treatment of mild persistent asthma with low doses of inhaled budesonide alone or in combination with formoterol. For the Oxis and Pulmicort Turbuhaler in the Management of Asthma (OPTIMA) international study group. Thorax 2000; Vol. 55, issue Suppl 3:s5.
-
- Barnes PJ, O'Byrne PM, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Oxis and Pulmicort turbuhaler in the management of asthma OPTIMA international study group. Treatment of mild persistent asthma with low doses of inhaled budesonide alone or in combination with formoterol. Thorax 2000;55(Suppl 3):A4.
-
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Budesonide and formoterol in mild persistent asthma compared with doubling the dose of budesonide ‐ a cost‐effectiveness analysis. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:517s.
-
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Economic results of adding formoterol to budesonide in mild persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:331s.
-
- O'Byrne PM, Barnes PJ, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: The OPTIMA randomized trial. American Journal of Respiratory & Critical Care Medicine 2001;164(8 pt 1):1392‐7. - PubMed
O'Byrne 2001b {published data only}
-
- Barnes PJ, O'Byrne PM, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Oxis and Pulmicort turbuhaler in the management of asthma OPTIMA international study group. Treatment of mild persistent asthma with low doses of inhaled budesonide alone or in combination with formoterol. Thorax 2000;55(Suppl 3):A4.
-
- Barnes PJ, O'Byrne PM, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Treatment of mild persistent asthma with low doses of inhaled budesonide alone or in combination with formoterol. For the Oxis and Pulmicort Turbuhaler in the Management of Asthma (OPTIMA) international study group. Thorax 2000; Vol. 55, issue Suppl 3:s5.
-
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Budesonide and formoterol in mild persistent asthma compared with doubling the dose of budesonide ‐ a cost‐effectiveness analysis. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:517s.
-
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Economic results of adding formoterol to budesonide in mild persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:331s.
-
- O'Byrne PM, Barnes PJ, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. American Journal of Respiratory & Critical Care Medicine 2001;164(8 pt 1):1392‐7. - PubMed
Pauwels 1997a {published data only}
-
- Pauwels R. Additive effects of inhaled formoterol and budesonide in reducing asthma exacerbations. Allergy 1998;53:20‐3. - PubMed
-
- Pauwels RA, Lofdahl CG, Postma DA, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine 1997;337(20):1405‐11. - PubMed
Pauwels 1997b {published data only}
-
- Pauwels R. Additive effects of inhaled formoterol and budesonide in reducing asthma exacerbations. Allergy 1998;53:20‐3. - PubMed
-
- Pauwels RA, Lofdahl CG, Postma DA, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine 1997;337(20):1405‐11. - PubMed
Pohunek 2006a {published data only}
-
- Pohunek P, Kuna P, Boeck K. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma [Abstract]. European Respiratory Journal 2004;24(Suppl 48):379s.
-
- Pohunek P, Kuna P, Jorup C, Boeck K. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatric Allergy and Immunology 2006;17(6):458‐65. - PubMed
-
- Pohunek P, Matulka M, Rybnicek O, Kopriva F, Honomichlova H, Svobodova T. Dose‐related efficacy and safety of formoterol (Oxis) Turbuhaler compared with salmeterol Diskhaler in children with asthma. Pediatric Allergy & Immunology 2004;15(1):32‐9. - PubMed
Pohunek 2006b {published data only}
-
- Pohunek P, Kuna P, Boeck K. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma [Abstract]. European Respiratory Journal 2004;24(Suppl 48):379s.
-
- Pohunek P, Kuna P, Jorup C, Boeck K. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatric Allergy and Immunology 2006;17(6):458‐65. - PubMed
-
- Pohunek P, Matulka M, Rybnicek O, Kopriva F, Honomichlova H, Svobodova T. Dose‐related efficacy and safety of formoterol (Oxis) Turbuhaler compared with salmeterol Diskhaler in children with asthma. Pediatric Allergy & Immunology 2004;15(1):32‐9. - PubMed
Price 2002 {published data only}
-
- Price D, Dutchman D, Mawson A, Bodalia B, Duggan S, Todd P, FLOW (Eformoterol in the management of mild asthma‐eformoterol Turboinhaler with budesonide Turbohaler) research group. Early asthma control and maintenance with eformoterol following reduction of inhaled corticosteroid dose. Thorax 2002;57(9):791‐8. - PMC - PubMed
-
- Price MJ, Briggs AH. Development of an economic model to assess the cost effectiveness of asthma management strategies. Pharmacoeconomics 2002; Vol. 20, issue 3:183‐94. - PubMed
-
- Price MJ, Sondhi S, Yan S, Nyth A, House K. Salmeterol/fluticasone propionate combination inhaler is more cost effective than fluticasone propionate in patients with asthma. European Respiratory Society 1999 Annual Congress; Oct 9‐13; Madrid, Spain. 1999.
Reddel 2007 {unpublished data only}
-
- Reddel HK, Peyters MJ, Wark PA, Sand IB, Jenkins CR. Comparison of the efficacy of Seretide and Flixotide when down‐titrating the inhaled corticosteroid dose. Respirology 2007;12(Suppl 1):A40.
Russell 1995 {published data only}
-
- Russell G, Williams DAJ, Weller P, Price JF. Salmeterol xinafoate on children on high dose inhaled steroids. Annals of Allergy, Asthma, and Immunology 1995;75:423‐8. - PubMed
-
- SALMP/AH91/D89 (GSK). A phase III, multi‐centre, double‐blind, placebo controlled, parallel group study assessing the efficacy and safety of inhaled salmeterol xinafoate (Serevent™) 50 micrograms BD via the Diskhaler™ when added to the existing treatment of moderate to severe asthmatic children. http://ctr.gsk.co.uk 2006 (accessed 20 June 2008).
SAM40008 {unpublished data only}
-
- SAM40008. A multicentre, randomised, double‐blind, parallel group comparison of the efficacy of Seretide bd and fluticasone propionate bd (both via diskus/accuhaler inhaler) when tapering the inhaled corticosteroid dose in asthmatic adults. http://www.ctr.gsk.co.uk 2004.
SAM40012 {unpublished data only}
-
- Dorinsky P, Emmett A, Sutton L. Reduced risk for asthma exacerbations in pediatric patients receiving salmeterol plus inhaled corticosteroids (ICS) versus ICS alone [Abstract]. European Respiratory Journal 2004;24(Suppl 48):308s.
-
- SAM40012. A multicentre, randomised, double‐blind, double‐dummy, parallel group comparison of three treatments: 1) salmeterol/fluticasone propionate (SFC) (50/100mcg strength) bd via DISKUS/ACCUHALER inhaler, 2) fluticasone propionate 200mcg bd via DISKUS/ACCUHALER inhaler, 3) fluticasone propionate 100mcg bd via DISKUS/ACCUHALER inhaler in children aged 4‐11 years with asthma. http://www.ctr.gsk.co.uk 2005.
SAS40024 {unpublished data only}
-
- Dorinsky P, Jones S, Kalberg C, Emmett A, Rickard K. Sustained protection against activity‐induced bronchospasm (AIB) during chronic treatment with the fluticasone propionate/salmeterol combination (FSC). American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A568.
-
- Dorinsky P, Kalberg C, Emmett A, Rickard K. Sustained protection against activity‐induced bronchospasm during chronic treatment with fluticasone/salmeterol combination. European Respiratory Journal 2002;20(Suppl 38):308s.
-
- SAS40024. A randomized, double‐blind, parallel‐group study evaluating the protective effects of the fluticasone propionate/salmeterol combination product (FSC, 100/50mcg BID via diskus) against bronchospasms induced by activity as measured by exercise challenge testing in adolescent and adult subjects who require chronic inhaled corticosteroid therapy for the treatment of persistent asthma. http://www.ctr.gsk.co.uk 2005.
SAS40036 {unpublished data only}
-
- Keonig S, Waitkus‐Edwards K, Yancey S, Prillaman B, Dorinsky P. Loss of asthma control when patients receiving fluticasone propionate/salmeterol 100/50 mcg Diskus® are stepped‐down to fluticasone propionate, salmeterol or montelukast alone. Journal of Allergy and Clinical Immunology 2004;113(2 (Suppl 1)):S94. [DOI: 10.1016/j.jaci.2003.12.325] - DOI
-
- SAS40036. A multicenter, randomized, double‐blind, double‐dummy, parallel group, 16‐week comparison of asthma control in adolescents and adults receiving either fluticasone propionate/salmeterol Diskus combination product 100/50mcg BID, fluticasone propionate Diskus 100mcg BID, salmeterol xinafoate Diskus 50mcg BID, or oral montelukast 10mg QD. http://www.ctr.gsk.co.uk 2005.
SAS40037 {unpublished data only}
-
- SAS40037. A multicenter, randomized, double‐blind, double‐dummy, parallel group, 16‐week comparison of asthma control in adolescents and adults receiving either fluticasone propionate/salmeterol Diskus (combination product 100/50mcg BID, fluticasone propionate Diskus 100mcg BID, salmeterol xinafoate Diskus 50mcg BID, or oral montelukast 10mg QD. http://www.ctr.gsk.co.uk 2005.
SD 037 0344a {unpublished data only}
-
- AstraZeneca (SD 037 0344). A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised, parallel group, phase III study to investigate the efficacy and safety of formoterol HFA pMDI compared with placebo and Oxis® Turbuhaler® in subjects with asthma. http://www.astrazenecaclinicaltrials.com 2003 (accessed 30 April 2008).
SD 037 0344b {unpublished data only}
-
- AstraZeneca (SD 037 0344). A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised, parallel group, phase III study to investigate the efficacy and safety of Formoterol HFA pMDI compared with placebo and Oxis® Turbuhaler® in subjects with asthma. http://www.astrazenecaclinicaltrials.com 2003 (accessed 30 April 2008).
SD 039 0349 {unpublished data only}
-
- AstraZeneca (SD 039 0349). Efficacy and safety of a fixed combination of budesonide/formoterol Turbuhaler® in inhaled steroid‐using asthmatic adults. http://www.astrazenecaclinicaltrials.com 1999 (accessed 30 April 2008).
SD 039 0714 {unpublished data only}
-
- AstraZeneca Pharmaceuticals. Efficacy and safety of budesonide/formoterol Turbuhaler® (160/4.5 mcg b.i.d. delivered dose) compared to budesonide Turbuhaler® (200 mcg b.i.d. metered dose) in steroid‐using asthmatic adolescent patients. A double‐blind, double‐dummy, randomised, parallel group, phase III, multicentre study. www.astrazenecaclinicaltrials.com 2005 (accessed 21 February 2006).
SD 039 0718 {unpublished data only}
-
- AstraZeneca Pharmaceuticals (SD 039 0718). A twelve‐week, randomized, double‐blind, double‐dummy trial of Symbicort® (40/4.5 mcg) versus its mono‐products (budesonide and formoterol) in asthmatic children aged six to fifteen years. http://www.astrazenecaclinicaltrials.com 2005 (accessed 4 January 2008).
SD 039 0719 {unpublished data only}
-
- AstraZeneca Pharmaceuticals (SD 039 0719). A six‐month, randomized, open‐label safety study of Symbicort® (160/4.5 mcg) compared to Pulmicort Turbuhaler® in asthmatic children aged 6 to 11 years. http://www.astrazenecaclinicaltrials.com 2005 (accessed 4 January 2008).
SD 039 0725a {unpublished data only}
-
- AstraZeneca Pharmaceuticals (SD 039 0725). A twelve‐week, randomized, double‐blind, double‐dummy, active‐controlled study of Symbicort® pMDI administered once daily in children and adolescents 6 to 15 years of age with asthma. http://www.astrazenecaclinicaltrials.com 2005 (accessed 4 January 2008).
SD 039 0725b {unpublished data only}
-
- AstraZeneca Pharmaceuticals (SD 039 0725). A twelve‐week, randomized, double‐blind, double‐dummy, active‐controlled study of Symbicort® pMDI administered once daily in children and adolescents 6 to 15 years of age with asthma. http://www.astrazenecaclinicaltrials.com 2005 (accessed 4 January 2008).
SD 039 0726a {unpublished data only}
-
- Ambrose H, Lawrance R, Goldman M. Beta‐adrenergic receptor Gly16Arg variation: effect on response to budesonide/formoterol or budesonide (post‐formoterol) in asthma patients. http://meeting.chestjournal.org. 2007 (accessed 27 June 2008).
-
- Berger WE, Bleecker ER, O'Dowd L, Miller CJ. Asthma control with once‐daily budesonide/formoterol (BUD/FM) pressurized metered‐dose inhaler. http://www.abstracts2view.com 2007 (accessed 26 June 2008).
-
- Bleecker ER, Berger WE, O'Dowd L, Miller CJ. Safety of once‐daily budesonide (BUD) and formoterol (FM) administered via one pressurized metered‐dose inhaler (pMDI) in patients with asthma. http://www.abstracts2view.com 2007 (accessed 27 June 2008).
-
- SD 039 0726 (AstraZeneca). A twelve‐week, randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled study of SYMBICORT® pMDI administered once daily in adults and adolescents with asthma. www.astrazenecaclinicaltrials.com 2005 (accessed 26 June 2008).
SD 039 0726b {unpublished data only}
-
- Ambrose H, Lawrance R, Goldman M. Beta‐adrenergic receptor Gly16Arg variation: Effect on response to budesonide/formoterol or budesonide (post‐formoterol) in asthma patients. http://meeting.chestjournal.org. 2007 (accessed 27 June 2008).
-
- Berger WE, Bleecker ER, O'Dowd L, Miller CJ. Asthma control with once‐daily budesonide/formoterol (BUD/FM) pressurized metered‐dose inhaler. http://www.abstracts2view.com 2007 (accessed 26 June 2008).
-
- Bleecker ER, Berger WE, O'Dowd L, Miller CJ. Safety of once‐daily budesonide (BUD) and formoterol (FM) administered via one pressurized metered‐dose inhaler (pMDI) in patients with asthma. http://www.abstracts2view.com 2007 (accessed 27 June 2008).
-
- SD 039 0726 (AstraZeneca). A twelve‐week, randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled study of SYMBICORT® pMDI administered once daily in adults and adolescents with asthma. www.astrazenecaclinicaltrials.com 2005 (accessed 26 June 2008).
SD 039 0728 {unpublished data only}
-
- O'Brien CD, Peters SP, Prenner BM, Martin P. Long‐term safety of budesonide/formoterol pressurized metered‐dose inhaler (BUD/FM pMDI) in asthma patients: adverse events and asthma exacerbations. http://www.abstracts2view.com 2007 (accessed 26 June 2008).
-
- O'Brien CD, Peters SP, Prenner BM, Martin P. Resource use with budesonide/formoterol pressurized metered‐dose inhaler (BUD/FM pMDI) versus BUD pMDI in asthma patients. www.astrazenecaclinicaltrials.com 2007 (accessed 26 June 2008).
-
- Peters SP, Prenner BM, Martin P, O'Brien CD. Long‐term effects on lung function of budesonide (BUD) and formoterol (FM) in one pressurized metered‐dose inhaler (BUD/FM pMDI) and BUD pMDI in patients with asthma. http://www.abstracts2view.com 2007 (accessed 26 June 2008).
-
- Prenner BM, Peters SP, Martin P, O'Brien CD. Long‐term control of asthma symptoms with budesonide/formoterol pressurized metered‐dose inhaler (BUD/FM pMDI) versus BUD pMDI. http://www.abstracts2view.com 2007 (accessed 26 June 2008).
-
- Prenner BM, Peters SP, Martin P, O'Brien CD. Safety pharmacodynamics (PD) of budesonide/formoterol (BUD/FM) pMDI in asthma patients. http://www.abstracts2view.com 2007 (accessed 26 June 2008).
SFA100314 {unpublished data only}
-
- GlaxoSmithKline (SFA100314). A stratified, multicenter, randomized, double‐blind, parallel group, 4‐week comparison of fluticasone propionate/salmeterol DISKUS combination product 100/50mcg BID versus fluticasone propionate DISKUS 100mcg BID in pediatric and in adolescent subjects with activity‐induced bronchospasm. http://www.ctr.gsk.co.uk 2007 (accessed 16 May 2008).
SFA100316 {unpublished data only}
-
- GlaxoSmithKline (SFA100316). A stratified, multicenter, randomized, double‐blind, parallel group, 4‐week comparison of fluticasone propionate/salmeterol DISKUS combination product 100/50mcg BID versus fluticasone propionate DISKUS 100mcg BID in pediatric and in adolescent subjects with activity‐induced bronchospasm. http://ctr.gsk.co.uk 2006 (accessed 30 April 2008).
SFCF4026 {unpublished data only}
-
- SFCF4026. Maintenance of asthma control in adults: comparison of three therapeutic strategies in patients whose asthma is controlled by a medium dose of inhaled corticosteroid and a long‐acting inhaled beta2‐agonist. http://www.ctr.gsk.co.uk 2005.
Shapiro 2000 {published data only}
-
- SFCA3003. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of salmeterol 50mcg BID and fluticasone propionate 250mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk 2004 (accessed 4 June 2008).
-
- Shapiro G, Lumry W, Wolfe J, Given J, White MV, Woodring A, et al. Combined salmeterol 50mcg and fluticasone propionate 350mcg in the Diskus device for the treatment of asthma. American Journal of Respiratory & Critical Care Medicine 2000;161:527‐34. - PubMed
Simons 1997 {published data only}
-
- Simons FE, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise‐induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics 1997;99(5):655‐9. - PubMed
SMS40012 {unpublished data only}
-
- SLMF 4002 (SMS40012). Efficacy and safety of salmeterol in patients with asthma controlled with inhaled corticosteroids. http://www.ctr.gsk.co.uk 2005.
Stelmach 2007 {published data only (unpublished sought but not used)}
-
- Stelmach I, Grzelewskia T, Bobrowska‐Korzeniowska M, Stelmach P, Kuna P. A randomized, double‐blind trial of the effect of anti‐asthma treatment on lung function in children with asthma. Pulmonary Pharmacology & Therapeutics 2007;20:691‐700. - PubMed
Tal 2002 {published data only}
-
- AstraZeneca (SD 039 0353). Efficacy and safety of budesonide/formoterol Turbuhaler® in a fixed combination in steroid‐using asthmatic children. http://www.astrazenecaclinicaltrials.com 1999 (accessed 30 April 2008).
-
- Tal A, Simon G, Vermeulen JH. Symbicort® (budesonide and formoterol in a single inhaler) is effective and well tolerated in children with asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
-
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatric Pulmonology 2002;34(5):342‐50. - PubMed
-
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Rapid and sustained improvements in lung function and symptom control with budesonide/formoterol in adolescent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:494s.
-
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Symbicort (budesonide and formoterol in a single inhaler) improves lung function in children with asthma. International Paediatric Respiratory and Allergy Congress, April 1‐4; Prague. 2001:85.
Teper 2005 {published data only}
-
- Teper AM, Zaragoza SM, Lubovich S, Rodriguez VA, Venalago C, Kofman CD, et al. Effect of fluticasone propionate (FP) with or without salmeterol (S) on bronchial reactivity (BR) in children with mild to moderate persistent asthma [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:[C47] [Poster: A5].
van der Molen 1997 {published data only}
-
- Molen T, Postma DS, Kraan J, Chapman K, Grossman R, Turner MO, et al. No influence of six months treatment with formoterol on airway hyperresponsiveness in asthma subjects using inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 1998;157(3 Suppl):A400.
-
- Molen T, Sears MR, Graaff CS, Postma DS, Meyboom‐de Jong B. Quality of life during formoterol treatment: comparison between asthma‐specific and generic questionnaires. European Respiratory Journal 1998;12(1):30‐4. - PubMed
Verberne 1998 {published data only}
-
- SLGB4014 (SLPT15). Placebo controlled study during one year comparing the addition of salmeterol with an increase of the dose of the inhaled corticosteroid in asthmatic children already on treatment with inhaled corticosteroids. http://ctr.gsk.co.uk 2006 (accessed 20 June 2008).
-
- Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerribijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. American Journal of Respiratory & Critical Care Medicine 1998;158:213‐19. - PubMed
Wallin 2003 {published data only}
-
- Sue‐Chu M, Wallin A, Wilson S, Ward J, Sandstrom T, Djukanovic R, et al. Bronchial biopsy study in asthmatics treated with low and high dose fluticasone propionate (FP) compared to low dose FP combined with salmeterol. European Respiratory Society 1999 Annual Congress Oct 9‐13; Madrid, Spain. 1999.
-
- Wallin A, Sandstrom T, Cioppa GD, Holgate S, Wilson S. The effects of regular inhaled formoterol and budesonide on preformed Th‐2 cytokines in mild asthmatics. Respiratory Medicine 2002;96(12):1021‐5. - PubMed
-
- Wallin A, Sue‐Chu M, Bjermer L, Ward J, Sandstrom T, Lindberg A, et al. Effect of inhaled fluticasone with and without salmeterol on airway inflammation in asthma. Journal of Allergy & Clinical Immunology 2003;112(1):72‐8. - PubMed
Weiler 2005 {published and unpublished data}
-
- SAS40025. A randomized, double‐blind, parallel‐group study evaluating the protective effects of the fluticasone propionate/salmeterol combination product (FSC 250/50mcg BID via diskus) against bronchospasms induced by activity as measured by exercise challenge testing in adolescent and adult subjects who require chronic inhaled corticosteroid therapy for the treatment of persistent asthma. http://www.clinicalstudyresults.org 2005 (accessed 4 November 2008).
-
- Weiler JM, Nathan RA, Rupp NT, Kalberg CJ, Emmett A, Dorinsky P. Effect of fluticasone/salmeterol via a single device on exercise‐induced bronchospasm in patients with persistent asthma. Annals of Allergy Asthma and Immunology 2005;94(1):65‐72. - PubMed
Zetterstrom 2001a {published data only}
-
- Zetterstrom O, Buhl R, Mellem H. Efficacy and safety of Symbicort® (budesonide and formoterol in a single inhaler) in adults with asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
-
- Zetterstrom O, Buhl R, Mellem H, Perpina M, Hedman J, O'Neill S, et al. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. European Respiratory Journal 2001;18(2):262‐8. - PubMed
-
- Zetterstrom O, Buhl R, Mellem H, Perpina M, Hedman J, O'Neill S, et al. The new single inhaler product containing both budesonide/formoterol improves asthma control in adults. European Respiratory Journal 2000; Vol. 16, issue Suppl 31:455s. - PubMed
-
- Zetterström O, Buhl R, Mellem H, Perpiñá M, Hedman J, O'Neill S, et al. Efficacy and safety of a new single inhaler product containing both budesonide and formoterol in adult asthma. European Respiratory Journal 2000; Vol. 16, issue 31:455s.
Zetterstrom 2001b {published data only}
-
- Zetterstrom O, Buhl R, Mellem H. Efficacy and safety of Symbicort® (budesonide and formoterol in a single inhaler) in adults with asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
-
- Zetterstrom O, Buhl R, Mellem H, Perpina M, Hedman J, O'Neill S, et al. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. European Respiratory Journal 2001;18(2):262‐8. - PubMed
-
- Zetterstrom O, Buhl R, Mellem H, Perpina M, Hedman J, O'Neill S, et al. The new single inhaler product containing both budesonide/formoterol improves asthma control in adults. European Respiratory Journal 2000;16(Suppl 31):455s. - PubMed
-
- Zetterström O, Buhl R, Mellem H, Perpiñá M, Hedman J, O'Neill S, et al. Efficacy and safety of a new single inhaler product containing both budesonide and formoterol in adult asthma. European Respiratory Journal 2000; Vol. 16, issue 31:455s.
Zimmerman 2004a {published data only}
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol turbuhaler(R) when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004;37(2):122‐7. - PubMed
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol Turbuhaler in 6‐11 year old children with asthma, not adequately controlled with inhaled corticosteroids. European Respiratory Society Annual Congress. 2002:P2734.
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol turbuhaler(r) compared with placebo, in children (6‐11 yr) with asthma poorly controlled with inhaled corticosteroids [abstract]. American Journal of Respiratory and Critical Care Medicine. 2002; Vol. 165, issue 8 Suppl:A746.
Zimmerman 2004b {published data only}
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol turbuhaler(R) when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004;37(2):122‐7. - PubMed
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol Turbuhaler in 6‐11 year old children with asthma, not adequately controlled with inhaled corticosteroids. European Respiratory Society Annual Congress. 2002:P2734.
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol turbuhaler(r) compared with placebo, in children (6‐11 yr) with asthma poorly controlled with inhaled corticosteroids [abstract]. American Journal of Respiratory and Critical Care Medicine. 2002; Vol. 165, issue 8 Suppl:A746.
References to studies excluded from this review
Aalbers 2004 {published data only}
-
- Aalbers R, Backer V, Kava TT, Omenaas ER, Sandstrom T, Jorup C, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed‐dose salmeterol/fluticasone in moderate to severe asthma. Current Medical Research & Opinion 2004;20(2):225‐40. - PubMed
-
- Aalbers R, Backer V, Kava TT, Welte T, Omenaas ER, Bergqvist PB, et al. Adjustable dosing with budesonide/formoterol reduces the rate of asthma exacerbations compared with fixed dosing salmeterol/fluticasone. European Respiratory Society. 2003:p‐2‐20.
-
- Aalbers R, Backer V, Kava TT, Welte T, Omenaas ER, Bergqvist PB, et al. Improvements in FEV1 are greater with budesonide/formoterol than with salmeterol/fluticasone. European Respiratory Society Annual Congress. 2003:P‐2‐19.
-
- Aalbers R, Backer V, Kava TT, Welte T, Omenaas ER, Bergqvist PBF, et al. Is well controlled asthma weeks a useful measure? Fewer exacerbations in patients treated with budesonide/formoterol than salmeterol/fluticasone. European Respiratory Society. 2003:P‐2‐18.
Adinoff 1998 {published data only}
-
- Adinoff AD, Schwartz HJ, Rickard KA, Yancey SW, Swearingen BE. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47(4):278‐84. - PubMed
Ankerst 2003 {published data only}
-
- Ankerst J, Persson G, Weibull E. Cardiovascular effects of a high dose of the budesonide/formoterol single inhaler in asthmatic patients. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:53s.
-
- Ankerst J, Persson G, Weibull E. Tolerability of a high dose of budesonide/formoterol in a single inhaler in patients with asthma. Pulmonary Pharmacology & Therapeutics 2003;16:147‐51. - PubMed
Anonymous 2003 {published data only}
-
- Anonymous. Flexible dosing of combination inhaler cuts asthma exacerbations. Pharmaceutical Journal 2003;271(7271):535.
Arvidsson 1991 {published data only}
-
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Svedmyr N, Wahlander L. Inhaled formoterol during one year in asthma: a comparison with salbutamol. European Respiratory Journal 1991;4(10):1168‐73. - PubMed
Aziz 1998 {published data only}
-
- Aziz I, Hall IP, McFarlane LC, Lipworth BJ. Beta2‐adrenoceptor regulation and bronchodilator sensitivity after regular treatment with formoterol in subjects with stable asthma. Journal of Allergy & Clinical Immunology 1998;101(3):337‐41. - PubMed
Aziz 1999a {published data only}
-
- Aziz I, Lipworth BJ. A bolus of inhaled budesonide rapidly reverses airway subsensitivity and beta2‐adrenoceptor down‐regulation after regular inhaled formoterol. Chest 1999;115(3):623‐8. - PubMed
Aziz 1999b {published data only}
-
- Aziz I, Lipworth BJ. In vivo effect of albuterol on methacholine‐contracted bronchi in conjunction with salmeterol and formoterol. Journal of Allergy & Clinical Immunology 1999;103(5 pt 1):816‐22. - PubMed
Aziz 2000 {published data only}
-
- Aziz I, Wilson AM, Lipworth BJ. Effects of formoterol (FM) and budesonide (BUD) alone or in combination (FM+BUD) on inflammatory markers in asthmatic patients. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:2854.
-
- Aziz I, Wilson AM, Lipworth BJ. Effects of once‐daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest 2000;118(4):1049‐58. - PubMed
Bacci 2002 {published data only}
-
- Bacci E, Franco A, Bartoli ML, Carnevali S, Cianchetti S, Dente FL, et al. Comparison of anti‐inflammatory and clinical effects of beclomethasone dipropionate and salmeterol in moderate asthma. European Respiratory Journal 2002;20(1):66‐72. - PubMed
Baker 1998 {published data only}
-
- Baker J, Yancey S, Kalberg C, Petrocella V, Emmett A, Bowers B, et al. Added salmeterol versus increased‐dose fluticasone in patients symptomatic on low‐dose fluticasone. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A406.
Baki 1998 {published data only}
-
- Baki A, Karaguzel G. Short‐term effects of budesonide, nedocromil sodium and salmeterol on bronchial hyperresponsiveness in childhood asthma. Acta Paediatrica Japonica 1998;40(3):247‐51. - PubMed
Baraniuk 1999 {published data only}
-
- Baraniuk J, Murray JJ, Nathan RA, Berger WE, Johnson M, Edwards LD, et al. Fluticasone alone or in combination with salmeterol vs triamcinolone in asthma. Chest 1999;116(3):625‐32. - PubMed
-
- Cook D, Srebro SH, Rogenes PR, Rickard K, Edwards L, Johnson MC. A comparison of the safety and efficacy of fluticasone, triamcinolone, and fluticasone plus salmeterol in patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A416.
-
- Johnson MC, Srebro SH, Rogenes PR, Rickard K, Edwards L. A comparison of physician‐rated and patient‐rated outcomes in a study with fluticasone, triamcinolone, and fluticasone plus salmeterol. American Journal of Respiratory & Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A414.
Bateman 1998 {published data only}
-
- Bateman ED, Britton M, Carrillo J, Almeida J, Wixon C. Salmeterol/fluticasone combination inhaler. A new, effective and well tolerated treatment for asthma. Clinical Drug Investigation 1998;16(3):193‐201. - PubMed
Bateman 2003a {published data only}
-
- Bateman ED, Bantje TA, Gomes M, Toumbis M, Huber R, Eliraz A, et al. Symbicort (budesonide and formoterol in a single inhaler) is a more effective treatment than fluticasone in asthma patients. Annual Thoracic Society 97th International Conference. 2001.
-
- Bateman ED, Bantje TA, Gomes MJ, Toumbis MG, Huber RM, Naya I, et al. Combination therapy with a single inhaler budesonide/formoterol compared with high dose fluticasone propionate alone in patients with moderate persistent asthma. American Journal of Respiratory Medicine 2003;2(3):275‐81. - PubMed
-
- Bateman ED, Bantje TA, Joao Gomes M, Toumbis M, Huber R, Eliraz A. Early and sustained benefits of budesonide and formoterol in a single inhaler vs fluticasone in moderate asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:157s.
-
- Bateman ED, Bantje TA, Joao Gomes M, Toumbis M, Huber R, Eliraz A. Early and sustained benefits of budesonide and formoterol in a single inhaler vs fluticasone in moderate asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:157s.
-
- Bateman ED, Bantje TA, Joao Gomes M, Toumbis M, Huber R, Eliraz A, et al. Symbicort (budesonide/eformoterol) Turbohaler controls asthma more effectively than fluticasone Diskus. Thorax 2001; Vol. 56, issue Suppl 3:iii 63.
Bateman 2003b {published data only}
-
- Bateman ED, Akveld M, Ho M. Greater responder rate to fluticasone propionate/salmeterol combination over montelukast plus fluticasone in asthma. American Thoracic Society 99th International Conference. 2003:B036 Poster H90.
Behling 1999 {published data only}
-
- Behling B, Matthys H. Comparison of efficacy and safety of formoterol with terbutaline in children with mild to moderate asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:369.
Bensch 2002 {published data only}
-
- Bensch G, Berger WE, Blokhnn M, Socolovshy AL, Thomson MH, Till MD, et al. One year efficacy and safety of inhaled formoterol dry powder in children with persistent asthma. Annals of Allergy, Asthma, and Immunology 2002;89:180‐90. - PubMed
Berger 2001 {published data only}
-
- Berger W, Bensch G, Blokhin BM, Socolovsky AL, Thompson MH, Till D. Addition of formoterol (Foradil®) improves lung function and symptoms in children with persistent asthma not controlled by inhaled corticosteroids. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Berggren 2001 {published data only}
-
- Berggren F, Ekstrom T. A cost‐effectiveness study comparing the as‐needed use of formoterol (Oxis) and terbutaline (Bricanyl) in patients with moderate to severe asthma. Respiratory Medicine 2001;95(9):753‐8. - PubMed
Bergmann 2004 {published data only}
-
- Bergmann KC, Lindemann L, Braun R, Steinkamp G. Salmeterol/fluticasone propionate (50/250 mug) combination is superior to double dose fluticasone (500 mug) for the treatment of symptomatic moderate asthma: a prospective, double‐blind trial. Swiss Medical Weekly 2004;134(3‐4):50‐8. - PubMed
Bernstein 2002 {published data only}
-
- Bernstein IL. Beta2‐agonists: Deja vu all over again: the second‐generation controversy. Chest 2002; Vol. 122, issue 3:763‐5. - PubMed
Bessmertny 2002 {published data only}
-
- Bessmertny O, DiGregorio RV, Cohen H, Becker E, Looney D, Golden J, et al. A randomized clinical trial of nebulized magnesium sulfate in addition to albuterol in the treatment of acute mild‐to‐moderate asthma exacerbations in adults. Annals of Emergency Medicine 2002;39(6):585‐91. - PubMed
Bijl‐Hofland 2001 {published data only}
-
- Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C, Schayck CP. Inhaled corticosteroids, combined with long‐acting beta(2)‐agonists, improve the perception of bronchoconstriction in asthma. American Journal of Respiratory & Critical Care Medicine 2001;164(5):764‐9. - PubMed
Bjermer 2000 {published data only}
-
- Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening A, Haahtela T, et al. Montelukast or salmeterol combined with an inhaled steroid in adult asthma: design and rationale of a randomized, double‐blind comparative study (the IMPACT Investigation of Montelukast as a Partner Agent for Complementary Therapy‐trial). Respiratory Medicine 2000;94(6):612‐21. - PubMed
Bjermer 2002 {published data only}
-
- Bjermer L, Greening A, Haahtela T, Bousquet J, Holgate ST, Picado C, et al. Addition of montelukast or salmeterol to fluticasone in patients with uncontrolled asthma: results of the IMPACT trial. Chest 2002; San Diego, CA. 2002:434.
Bjermer 2003 {published data only}
Bloom 2003 {published data only}
-
- Bloom J, Calhoun W, Koenig S, Yancey S, Reilly D, Edwards L, et al. Fluticasone propionate/salmeterol 100/50mcg is inhaled steroid sparing in patients who require fluticasone propionate 250mcg for asthma stability. American Thoracic Society 99th International Conference. 2003:D034 Poster C33.
Boonsawat 2003 {published data only}
-
- Boonsawat W, Charoenratanakul S, Pothirat C, Sawanyawisuth K, Seearamroongruang T, Bengtsson T, et al. Formoterol (OXIS) Turbuhaler as a rescue therapy compared with salbutamol pMDI plus spacer in patients with acute severe asthma. Respiratory Medicine 2003;97(9):1067‐74. - PubMed
Booth 1993 {published data only}
Boskovska 2001 {published data only}
-
- Boskovska MI, Dokic D, Busletic‐Bozinovska K, Arbutina S, Goseva Z. Concomitant use of low‐dose inhaled corticosteroids and a long‐acting bronchodilator vis a vis doubling the dose of inhaled corticosteroid in asthma patients. European Respiratory Journal 2001, issue Suppl 33:98s.
Bouchard 2000 {published data only}
-
- Bouchard J, Arkinstall W, Tesarowski D. Efficacy of salmeterol and fluticasone propionate (FP) combination therapy versus FP alone in mild/moderate asthma. American Journal of Respiratory and Critical Care Medicine. 2000; Vol. 161, issue Suppl 3:A197.
Boulet 2003 {unpublished data only}
-
- Boulet LP, Chapman K, Roberts J, Watson EG. Efficacy of salmeterol/fluticasone propionate HFA MDI versus high dose fluticasone propionate HFA MDI in adolescent and adult asthma. European Respiratory Society Meeting. 2003.
Bouros 1999 {published data only}
-
- Bouros D, Bachlitzanakis N, Kottakis J, Pfister P, Polychronopoulos V, Papadakis E, et al. Foromoterol and beclomethasone versus higher dose beclomethasone as maintenance therapy in adult asthma. European Respiratory Journal 1999;14:627‐32. - PubMed
Brambilla 1994 {published data only}
-
- Brambilla C, Chastang C, Georges D, Bertin L. Salmeterol compared with slow‐release terbutaline in nocturnal asthma. A multicenter, randomized, double‐blind, double‐dummy, sequential clinical trial. French Multicenter Study Group. Allergy 1994;49(6):421‐6. - PubMed
Brambilla 2003 {published data only}
-
- Brambilla C, Gros V, Bourdeix I. Formoterol 12 mcg bid administered via single dry powder inhaler in adults with asthma suboptimally controlled with salmeterol or on demand salbutamol. Clinical Therapeutics 2003;25(7):2022‐36. - PubMed
Braniuk 1999 {published data only}
-
- Braniuk J, Murray JJ, Nathan RA, Berger WE, Johnson M, Edwards LD, et al. Fluticasone alone or in combination with salmeterol vs triamcinolone in asthma. Chest 1999;116(3):625‐32. - PubMed
Brenner 1988 {published data only}
-
- Brenner M, Berkowitz R, Marshall N, Strunk RC. Need for theophylline in severe steroid‐requiring asthmatics. Clinical Allergy 1988;18(2):143‐50. - PubMed
Britton 1992 {published data only}
-
- Britton MG, Earnshaw JS, Palmer JBD. A twelve month comparison of salmeterol with salbutamol in asthmatic patients. European Respiratory Journal 1992;5(9):1062‐7. - PubMed
Britton 1998 {published data only}
-
- Britton MG, Carrillo T, Almeida J, Wixon C. Combined Serevent™ and fluticasone propionate (50/100µg strength) bd via one Diskus™ (Accuhaler™) inhaler compared with salmeterol 50µg and fluticasone propionate 100µg bd via two separate Diskus inhalers. American Journal of Respiratory & Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A415.
Brogden 1991 {published data only}
-
- Brogden RN, Faulds D. Salmeterol xinafoate. A review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs 1991;42(5):895‐912. - PubMed
Buchvald 2003 {published data only}
-
- Buchvald F, Bisgaard H. Comparisons of the complementary effect on exhaled nitric oside of salmeterol vs montelukast in asthmatic children taking regular budesonide. Annals of Allergy, Asthma & Immunology 2003;91(3):309‐13. - PubMed
Busse 1999 {published data only}
-
- Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. Journal of Allergy & Clinical Immunology 1999;103(6):1075‐80. - PubMed
Busse 2003a {published data only}
-
- Busse W, Koenig SM, Oppenheimer J, Sahn SA, Yancey SW, Reilly D, et al. Steroid‐sparing effects of fluticasone propionate 100mcg and salmeterol 50mcg administered twice daily in a single product in patients previously controlled with fluticasone propionate 250mcg administered twice daily. Journal of Allergy & Clinical Immunology 2003;111(2):57‐65. - PubMed
-
- Sahn S, Yancey S, Reilly D, Edwards L, Rickard K, Dorinsky P. The fluticasone propionate/salmeterol (FSC) combination product 100/50 mcg BID is steroid sparing in patients who require FP250 mcg BID for asthma stability . Chest 2002; San Diego, CA. 2002.
Busse 2003b {published data only}
-
- Busse W, Koenig SM, Oppenheimer J, Sahn SA, Yancey SW, Reilly D, et al. Steroid‐sparing effects of fluticasone propionate 100mcg and salmeterol 50mcg administered twice daily in a single product in patients previously controlled with fluticasone propionate 250mcg administered twice daily. Journal of Allergy & Clinical Immunology 2003;111(2):57‐65. - PubMed
Byrnes 2000 {published data only}
Calhoun 2001 {published data only}
-
- Calhoun WJ, Nelson HS, Nathan RA, Pepsin PJ, Kalberg C, Emmett A, et al. Comparison of fluticasone propionate‐salmeterol combination therapy and montelukast in patients who are symptomatic on short‐acting 2‐agonists alone. American Journal of Respiratory & Critical Care Medicine 2001;164(5):759‐63. - PubMed
Calverley 2002 {published data only}
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components in COPD. American Journal of Respiratory & Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A226.
Castle 1993 {published data only}
Cazzola 2000 {published data only}
-
- Cazzola M, Lorenzo G, Perna F, Calderaro F, Testi R, Centanni S. Additive effects of salmeterol and fluticasone or theophylline in COPD. Chest 2000;118(6):1576‐81. - PubMed
Chan 2001 {published data only}
-
- Chan JS, Cowie RL, Lazarenko GC, Little C, Scott S, Ford GT. Comparison of intramuscular betamethasone and oral prednisone in the prevention of relapse of acute asthma. Canadian Respiratory Journal. 2001;8(3):147‐52. - PubMed
Chapman 1999 {published data only}
-
- Chapman KR, Ringdal N, Backer V, Palmqvist M, Saarelainen S, Briggs M. Salmeterol and fluticasone propionate (50/250 mug) administered via combination Diskus inhaler: as effective as when given via separate Diskus inhalers. Canadian Respiratory Journal 1999;6(1):45‐51. - PubMed
Cheer 2003 {published data only}
-
- Cheer SM, Warner GT, Easthope SE. Formoterol delivered by Turbuhaler: in pediatric asthma. Paediatric Drugs 2003;5(1):63‐8. - PubMed
Cloosterman 2001 {published data only}
-
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta(2)‐agonists in allergic asthmatic patients. Chest 2001;119(5):1306‐15. - PubMed
Condemi 1999 {published data only}
-
- Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Annals of Allergy, Asthma, and Immunology 1999;82:383‐9. - PubMed
Condemi 2001 {published data only}
-
- Condemi JJ. Comparison of the efficacy of formoterol and salmeterol in patients with reversible obstructive airway disease: a multicenter, randomized, open‐label trial. Clinical Therapeutics 2001;23(9):1529‐41. - PubMed
Cook 2001 {published data only}
-
- Cook CK, Prillaman BA, House KW, Rickard KA, Shah TP. Concurrent use of salmeterol/fluticasone propionate Diskus® powder combination product and fluticasone propionate aqueous nasal spray does not adversely affect HPA‐axis function. Annals of Allergy, Asthma, and Immunology 2001;86:98.
Corren 2007 {published and unpublished data}
-
- AstraZeneca (SD 039 0617). A twelve week, randomized, double‐blind, double‐dummy, placebo‐controlled trial of SYMBICORT® pMDI (80/4.5 mcg) versus its mono‐products (budesonide and formoterol) in children and adults with asthma. www.astrazenecaclinicaltrials.com 2007 (accessed 15 May 2008).
-
- Corren J, Korenblat PE, Miller CJ, O'Brien CD, Mezzanotte WS. Twelve‐week, randomized, placebo‐controlled, multicenter study of the efficacy and tolerability of budesonide and formoterol in one metered‐dose inhaler compared with budesonide alone and formoterol alone in adolescents and adults with asthma. Clinical Therapeutics 2007;29(5):823‐43. - PubMed
Crompton 1999 {published data only}
-
- Crompton GK, Ayres JG, Basran G, Schiraldi G, Brusasco V, Eivindson A, et al. Comparison of oral bambuterol and inhaled salmeterol. American Journal of Respiratory & Critical Care Medicine 1999;159(3):824‐8. - PubMed
Currie 2002 {published data only}
-
- Currie GP, Stenback S, Lipworth BJ. Dissociation in effects of fluticasone but not fluticasone/salmeterol on lung function and airway hyperresponsiveness in mild persistent asthma. European Respiratory Society Annual Congress. 2002:P2391.
Currie 2003a {published data only}
-
- Currie GP, Lee DK, Haggart K, Bates CE, Lipworth BJ. Effects of montelukast on surrogate inflammatory markers in corticosteroid‐treated patients with asthma. American Journal of Respiratory and Critical Care Medicine 2003;167(9):1232‐8. - PubMed
Currie 2003b {published data only}
-
- Currie GP, Bates CE, Lee DK, Jackson CM, Lipworth BJ. Effects of fluticasone plus salmeterol versus twice the dose of fluticasone in asthmatic patients. European Journal of Clinical Pharmacology 2003;59(1):11‐15. - PubMed
Currie 2003c {published data only}
D'Alonzo 1994 {published data only}
-
- D'Alonzo GE, Nathan RA, Henochowicz, Morris RJ, Ratner P, Rennard SI. Salmetrol xinafoate as maintenance therapy compared with albuterol in patients with asthma. JAMA 1994;271:1412‐16. - PubMed
Dahl 1989 {published data only}
-
- Dahl R, Pedersen B, Hagglof B. Nocturnal asthma: effect of treatment with oral sustained‐release terbutaline, inhaled budesonide, and the two in combination. Journal of Allergy & Clinical Immunology. 1989;83(4):811‐5. - PubMed
Dahl 1991 {published data only}
-
- Dahl R, Earnshaw JS, Palmer JBD. Salmeterol: a four week study of a long‐acting beta‐adrenoceptor agonist for the treatment of reversible airways disease. European Respiratory Journal 1991;4(10):1178‐84. - PubMed
Dal Negro 2001a {published data only}
-
- Dal Negro R, Micheletto C, Tognella S, Trevisan F, Pomari C. Short‐term bronchodilation following salmeterol 50mcg and combined salmeterol + fluticasone p(50/250mcg) via Diskus: a randomized, double blind cross‐over study in reversible airway obstruction. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Dal Negro 2001b {published data only}
-
- Dal Negro RW, Micheletto C, Pomari C, Trevisan F, Tognella S. The combination salmeterol (S) + fluticasone propionate (F) in mild‐to‐moderate asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:426s.
Dal Negro 2002a {published data only}
-
- Dal Negro R, Micheletto C, Trevison F, Tognells S, Pomari C, Spencer S. Salmeterol & fluticasone 50µg/250µg bid vs salmeterol 50µg bid and vs placebo in the long‐term treatment of COPD. American Journal of Respiratory & Critical Care Medicine 2002, issue Suppl 8:A228.
Dal Negro 2002b {published data only}
-
- Dal Negro RW, Micheletto C, Tognella S, Trevisan F, Pomari C. Is the sequence of salmeterol (SM) and fluticasone p. (FP) dry powder inhalation crucial in persistent mild asthma?. European Respiratory Society Annual Congress. 2002:P396.
Davis 2001 {published data only}
-
- Davis ES, Bowers B, Pepsin P, Kalberg C, Dorinsky P. The impact of fluticasone propionate/salmeterol combination product compared to oral montelukast on asthma related quality of life. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Del Rio‐Navarro 2001a {published data only}
-
- Del‐Rio‐Navarro BE, Sienra‐Monge JJL, Alvarez‐Amador M, Reyes‐Ruiz N, Arevalo‐Salas A, Berber A. Serum potassium levels, CPK‐MB and ECG in children suffering asthma treated with beclomethasone or beclomethasone‐salmeterol. Allergologia et Immunopathologia 2001;29(1):16‐21. - PubMed
Del‐Rio‐Navarro 2001b {published data only}
-
- Rio BE, Corona L, Fregosa R, Berber A, Magana J, Sienra‐Monge JL. Effect of salmeterol and salmeterol plus beclomethasone on the saliva flow and saliva IgA levels on patients with chronic moderate persistent asthma (CMPA). Journal of Allergy and Clinical Immunology 2001; Vol. 107, issue 2:s11. - PubMed
-
- Del‐Rio‐Navarro BE, Coron‐Hernandez L, Fragoso‐Rios R, Berber A, Torres‐Alcantara S, Cuairan‐Ruidiaz V, et al. Effect of salmeterol and salmeterol plus beclomethasone on saliva flow and IgA in patients with moderate‐persistent chronic asthma. Annals of Allergy, Asthma and Immunology 2001;87(5):420‐3. - PubMed
Dempsey 2000a {published data only}
-
- Dempsey OJ, Wilson AM, Sims EJ, Lipworth BJ. Additive anti‐inflammatory effects of montelukast but not salmeterol in asthmatics suboptimally controlled on inhaled steroids. American Journal of Respiratory and Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A198.
Dempsey 2000b {published data only}
-
- Dempsey OJ, Wilson AM, Sims EJ, Mistry C, Lipworth BJ. Additive bronchoprotective and bronchodilator effects with single doses of salmeterol and montelukast in asthmatic patients receiving inhaled corticosteroids. Chest 2000;117(4):950‐3. - PubMed
Dente 2001a {published data only}
-
- Dente FL, Scuotri L, Bacci E, Franco A, Giannini D, Taccola M, et al. Effects of combined treatment ‐ fluticasone plus salmeterol ‐ on allergen‐induced asthmatic responses. American Journal of Respiratory and Critical Care Medicine. 2001; Vol. 463, issue Suppl 5:A419.
Dente 2001b {published data only}
-
- Dente FL, Scuotri L, Bacci E, DeSanctis M, Franco A, Giannini D, et al. Combined treatment with fluticasone plus salmeterol protects against allergen‐induced asthmatic responses better than each drug alone. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:349s.
Dicpinigaitis 2002 {published data only}
-
- Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough‐variant asthma. Journal of Asthma 2002;39(4):291‐7. - PubMed
Didier 1997 {published data only}
-
- Didier A, Campos Oriola R. A two‐month comparison of salmeterol/beclomethasone and slow‐release terbutaline/budesonide in moderate asthma management. Clinical Drug Investigation 1997;14(1):1‐11.
Djordjevic 1999 {published data only}
-
- Djordjevic D, Zickovic D, Stankovic I, Pejcic T, Ducic J, Rancic M, et al. Comparative study of three months treatment in combination of salmeterol and beclomethasone dipropionate (BDP) with doubling the dose of BDP in mild asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:p849.
Dorinsky 2001a {published data only}
-
- Dorinsky PM, Kalberg C, Pepsin P, Emmett A, Rickard K. Greater onset of improvement in clinical efficacy measures with first line use of the fluticasone/salmeterol combination product compared to montelukast. Annual Thoracic Society 97th International Conference; San Francisco CA May 18‐23. 2001.
Dorinsky 2001b {published data only}
-
- Dorinsky P, Kalberg C, Pepsin P, Emmett A, Bowers B, Rickard K. The fluticasone/salmeterol combination product is superior to montelukast as first‐line asthma control. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:263s.
Eliraz 2001 {published data only}
-
- Eliraz A, Ramirez‐Rivera A, Ferranti P, Holzer R, Garcia JM, Turcotte C, et al. Similar efficacy following four weeks treatment of asthmatics with formoterol 12 mcg BD delivered by two different dry powder inhalers; differences in inhaler handling. International Journal of Clinical Practice 2001;55(3):164‐70. - PubMed
Eliraz 2002a {published data only}
-
- Eliraz A, Fritscher CC, Perez CMR, Boonsawat W, Nang AN, Bardin P, et al. Symbicort® (budesonide/formoterol) achieves more rapid control of asthma that fluticasone in patients with mild asthma. American Journal of Respiratory & Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A567.
Eliraz 2002b {published data only}
-
- Eliraz A, Fritscher CC, Perez CMR, Boonsawat W, Nang AN, Bardin P, et al. Budesonide and formoterol in a single inhaler quickly gains asthma control compared with fluticasone propionate in mild asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:48s.
Ericsson 2001a {published data only}
-
- Ericsson K, Bantje TA, Huber H, Borg S. Symbicort® Turbuhaler® is more cost effective than fluticasone Diskus™ in the treatment of asthma. Annual Thoracic Society 97th International Conference; San Francisco CA , May 18‐23. 2001.
Ericsson2001b {published data only}
-
- Ericsson K, Bantje TA, Huber R, Borg S, Anderson F. Cost‐effectiveness of budesonide and formoterol in a single inhaler compared to fluticasone in the treatment of asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:157s.
Everden 2002 {published data only}
-
- Everden P, Lloyd A, Hutchinson J, Plumb J. Cost‐effectiveness of eformoterol Turbohaler(R) versus salmeterol Accuhaler(R) in children with symptomatic asthma. Respiratory Medicine 2002;96(4):250‐8. - PubMed
Faurschou 1994 {published data only}
-
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;49(40):827‐32. - PubMed
Faurschou 1996 {published data only}
-
- Faurschou P, Steffensen I, Jacques L. Effect of addition of inhaled salmeterol to the treatment of moderate‐to‐severe asthmatics uncontrolled on high‐dose inhaled steroids. European Respiratory Journal 1996;9(9):1885‐90. - PubMed
Fish 2000 {published data only}
-
- Fish J, Boone R, Emmett A, Yancey S, Knobil K, Rickard K. Salmeterol added to inhaled corticosteroids (ICS) provides greater asthma control compared to montelukast. American Journal of Respiratory & Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A203.
Fish 2001 {published data only}
-
- Fish JE, Israel E, Murray JJ, Emmett A, Boone R, Yancey SW, et al. Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest 2001;120(2):423‐30. - PubMed
Fitzpatrick 1990 {published data only}
Fowler 2002 {published data only}
-
- Fowler SJ, Currie PC, Lipworth BJ. Step down therapy with low dose fluticasone‐salmeterol combination or medium dose hydrofluoroalkane 134a‐beclomethasone alone. Journal of Allergy & Clinical Immunology 2002;109(6):929‐35. - PubMed
Fuglsang 1995 {published data only}
-
- Fuglsang G, Agertoft L, Vikre‐Jorgensen J, Pedersen S. Influence of budesonide on the response to inhaled terbutaline in children with mild asthma. Pediatric Allergy and Immunology 1995;6(2):103‐8. - PubMed
Gabrijelcic 2004 {published data only}
-
- Gabrijelcic J, Casas A, Rabinovich RA, Roca J, Barbera JA, Chung KF, et al. Formoterol protects against platelet‐activating factor‐induced effects in asthma. European Respiratory Journal. 2004;23(1):71‐5. - PubMed
Giannini 1996 {published data only}
-
- Giannini D, Carletti A, Dente FL, Testi R, Bacci D, Bancalari L, et al. Effect of inhaled beclomethasone dipropionate (BDP) on tolerance to salmeterol (S) in allergen induced bronchoconstriction. European Respiratory Journal 1996; Vol. 9, issue Suppl 23:272s.
Giannini 1998a {published data only}
-
- Giannini D, Franco A, Bacci E, Conti I, Dente FL, Kotopulos C, et al. Long‐term treatment with salmeterol and inhaled corticosteroids does not induce tolerance to the protective effect of salmeterol on allergen challenge. American Journal of Respiratory & Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A414.
Giannini 1998b {published data only}
-
- Giannini D, Franco A, Bacci E, Conti I, Dente FL, Kotopulos C, et al. One‐week regular treatment with salmeterol induces tolerance to the protective effect of salmeterol on allergen challenge only in subjects not regularly treated with salmeterol and inhaled corticosteroid. European Respiratory Journal 1998;12(Suppl 28):156s.
Giannini 1999 {published data only}
-
- Giannini D, Bacci E, Dente FL, Franco A, Vagaggini B, Testi R, et al. Inhaled beclomethasone dipropionate reverts tolerance to the protective effect of salmeterol on allergen challenge. Chest 1999;115(3):629‐34. - PubMed
Giannini 2000 {published data only}
-
- Giannini D, Franco A, Bacci E, Dente FL, Taccola M, Vagaggini B, et al. The protective effect of salbutamol inhaled using different devices on methacholine bronchoconstriction. Chest 2000;117(5):1319‐23. - PubMed
Giannini 2001 {published data only}
-
- Giannini D, Tonelli M, Franco A, Bacci E, Conti I, Dente FL, et al. Tolerance to the protective effect of salmeterol on allergen challenge in mild untreated asthmatics and in moderate asthmatics on inhaled corticosteroid treatment.. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:103s.
Giannini 2002a {published data only}
-
- Giannini D, Tonelli M, Franco A, Bacci E, Conti I, Dente FL, et al. Tolerance to the protective effect of salmeterol + fluticasone combination (50/250 µg) on allergen challenge in mild untreated asthmatics. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A566.
Giannini 2002b {published data only}
-
- Giannini D, Franco A, Bacci E, Conti I, Dente FL, Kotopulos C, et al. One‐week regular treatment with salmeterol induces tolerance to the protective effect of salmeterol on allergen challenge only in subjects not regularly treated with salmeterol and inhaled corticosteroid. European Respiratory Journal 1998;12(Suppl 28):156s.
Gizycki 2000 {published data only}
-
- Gizycki MJ, Venge P, Dahl R, Jeffery PK. Comparison of the effects of six weeks treatment with fluticasone or salmeterol on the late phase response (LPR) in mild asthma ‐ a bronchial biopsy study. American Journal of Respiratory and Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A203.
Gold 2001 {published data only}
-
- Gold M, Jõgi R, Mulder PGH, Akveld MLM. Salmeterol/fluticasone propionate combination 50/100µg bid is more effective than fluticasone propionate 100µg bid plus montelukast 10 mg once daily in reducing exacerbations. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:262s.
Green 2002 {published data only}
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Wardlaw AJ, et al. A placebo controlled comparison of formoterol, montelukast or higher dose of inhaled corticosteroids in subjects with symptomatic asthma despite treatment with low dose inhaled corticosteroids. Thorax 2002; Vol. 57, issue Suppl III:iii 11.
Greening 1994 {published data only}
-
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher‐dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344:219‐24. - PubMed
Grosclaude 2003 {published data only}
-
- Grosclaude M, Cerruti JL, Delannay B, Herent M, Spilthooren F, Desfougeres JL. The fixed fluticasone/salmeterol gives better control of asthma than the association of beclomethasone dipropionate‐montelukast. Allergie et Immunologie 2003;35(9):356‐62. - PubMed
Grzelewska‐Rzymowska 2003 {published data only}
-
- Grzelewska‐Rzymowska I, Malolepszy J, Molina M, Sladek K, Zarkovic J, Siergiejko Z. Equivalent asthma control and systemic safety of inhaled budesonide delivered via HFA‐134a or CFC propellant in a broad range of doses. Respiratory Medicine 2003;97(Suppl D):S10‐S19. - PubMed
Gustafsson 1994 {published data only}
-
- Gustafsson PM, Von BA, Jenkins MM. Salmeterol 50 mug twice daily in the treatment of mild‐to‐moderate asthma in childhood ‐ a comparison of two inhalation devices. European Journal of Clinical Research 1994;5:63‐73.
Hasani 2003 {published data only}
-
- Hasani A, Toms N, O'Connor J, Dilworth JP, Agnew JE. Effect of salmeterol xinafoate on lung mucociliary clearance in patients with asthma. Respiratory Medicine 2003;97(6):667‐71. - PubMed
Haughney 2002 {published data only}
-
- Haughney J, Price D, Rosen JP, Kennelly J. Adjustable maintenance treatment with budesonide/formoterol combination rapidly improves and maintains quality of life in asthma patients. European Respiratory Society Annual Congress. 2002:P379.
Heuck 1999 {published data only}
-
- Heuck C, Heickendorff L, Wolthers OD, Sygehus S. Short term growth and collagen turnover in asthmatics treated inhaled formoterol and budesonide. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:364.
Heuck 2000 {published data only}
Hyland 1995 {published data only}
Ind 2002a {published data only}
-
- Ind P, Haughney J, Price D, Rosen JP, Kennelly J. Managed adjustable dosing of budesonide/formoterol combination provides equivalent asthma control to fixed dosing at a lower overall dose. European Respiratory Society Annual Congress. 2002:P2450.
Ind 2002b {published data only}
-
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002;20(4):859‐66. - PubMed
Ind 2003a {published data only}
-
- Haughney J, Price D, Rosen JP, Kennelly J. Adjustable maintenance treatment with budesonide/formoterol combination rapidly improves and maintains quality of life in asthma patients. European Respiratory Society Annual Congress. 2002:P379.
-
- Ind PW, Price D, Haughney J, Rosen JP. Adjustable dosing with budesonide/formoterol in a single inhaler (Symbicort(r)) provides similarly effective treatment of asthma compared with fixed dosing but at a lower overall dose. American Thoracic Society 99th International Conference. 2003:D034 Poster C38.
Isabelle 2001 {published data only}
-
- Isabelle P, Bjamer D, Neuparth N, Desfougeres JL. Efficacy and safety of salmeterol/fluticasone combination 50/100mug bd via two different powder devices in children. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Jeffery 2002 {published data only}
-
- Jeffery PK, Venge P, Gizycki MJ, Egerod I, Dahl R, Faurschou P. Effects of salmeterol on mucosal inflammation in asthma: a placebo‐controlled study. European Respiratory Journal 2002;20(6):1378‐85. - PubMed
Jenkins 1995 {published data only}
-
- Jenkins M. Clinical evaluation of CFC‐free metered dose inhalers. Journal of Aerosol Medicine: Deposition, Clearance, and Effects in the Lung 1995;8(Suppl 1):s41‐s47. - PubMed
Jenkins 2000 {published data only}
-
- Becker I, Kielborn A, Price MJ, Volmer T, Lloyd AC. Cost‐effectiveness of salmeterol/fluticasone combination product and budesonide in asthma patients in Germany. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:854.
-
- Jenkins C, Woolcock A, James M. Superior overall control of moderate to severe asthma with salmeterol/fluticasone propionate (FP) combination (50/250 mcg bd) compared with three‐fold‐higher dose of budesonide (800mcg bd). European Respiratory Journal. 2000; Vol. 16, issue Suppl 31:456s.
-
- Jenkins C, Woolcock AJ, Saarelainen P, Lundbaack B, James MH. Salmeterol/fluticasone propionate combination therapy 50/250mcgs twice daily is more effective than budesonide 800 twice daily in treating moderate to severe asthma. Respiratory Medicine 2000;94:715‐23. - PubMed
-
- Lundback B, Jenkins C, Price MJ, Thwaites RM. Cost‐effectiveness of salmeterol/fluticasone propionate combination product 50/250 microgram twice daily and budesonide 800 microgram twice daily in the treatment of adults and adolescents with asthma. Respiratory Medicine 2000;94(7):724‐32. - PubMed
-
- Lundback B, Ronmark E, Jonsson AC, et al. Treatment effectiveness and exacerbations during one year with Seretide compared to fluticasone propionate and salmeterol in mild to moderate asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:176s.
Jenkins 2002 {published data only}
-
- Jenkins C. Combination therapy with fluticasone and salmeterol for symptomatic asthma produces similar benefits when given by Accuhaler in a single or two separate devices over 24 weeks. Respirology 2002; Vol. 7, issue Suppl:A20. [P22]
Johansson 2001 {published data only}
-
- Johansson G, McIvor RA, D'Ambrosio FP, Gratziou C, James MH. Comparison of salmeterol/fluticasone propionate combination with budesonide in patients with mild‐to‐moderate asthma. Clinical Drug Investigation 2001;21(9):633‐42.
Jones 1994 {published data only}
Juniper 1995 {published data only}
-
- Juniper EF, Johnston PR, Borkhoff CM, Guyatt GH, Boulet LP, Haukioja A. Quality of life in asthma clinical trials: comparison of salmeterol and salbutamol. American Journal of Respiratory & Critical Care Medicine 1995;151(1):66‐70. - PubMed
Juniper 1999 {published data only}
-
- Juniper EF, Svenson K, O'Byrne PM, Barnes PJ, Bauer CA, Lofdahl CG, et al. Asthma quality of life during 1 year of treatment with budesonide with or without formoterol. European Respiratory Journal 1999;14:1038‐43. - PubMed
Kaik 2002 {published data only}
-
- Kaik G, Kottakis I, Anagnostopoulou O, Sichletidis L, Bachlitzanakis N, D'Amato M, et al. Sequential flexible therapy with formoterol (Foradil(r)) plus budesonide (Miflonide(r)) versus a fixed combination of salmeterol and fluticasone (Seretide(r)) in asthma self‐management. European Respiratory Society Annual Congress. 2002.
Kalberg 1998 {published data only}
-
- Kalberg CJ, Nelson H, Yancey S, Petrocella V, Emmett AH, Rickard KA, et al. A comparison of added salmeterol versus increased‐dose fluticasone in patients symptomatic on low‐dose fluticasone [Abstract]. Journal of Allergy & Clinical Immunology 1998;101(Suppl):S6.
-
- Knobil K, Kalberg C, Emmett A, Rickard K. Adding salmeterol is more effective than increasing the dose of fluticasone for patients with asthma who are symptomatic on low dose fluticasone. European Respiratory Journal. 1998; Vol. 12, issue Suppl 29:19s, P160.
Kalra 1996 {published data only}
-
- Kalra S, Swystun VA, Bhagat R, Cockcroft DW. Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest 1996;109(4):953‐6. - PubMed
Karaman 2007 {published data only (unpublished sought but not used)}
-
- Karaman O, Arli O, Uzuner N, Islekel H, Babayigit A, Olmez D, et al. The effectiveness of asthma therapy alternatives and evaluating the effectiveness of asthma therapy by interleukin‐13 and interferon gamma levels in children. Allergy & Asthma Proceedings 2007;28(2):204‐9. - PubMed
Kardos 2001 {published data only}
-
- Kardos P, Bruggenjurgen B, Martin A, Meyer‐Sabellek W, Richter K, Vogelmeier C, et al. Treatment of bronchial asthma using a new adjustable combination treatment plan: Asthma Control Plan (ATACO). Pneumologie 2001;55(5):253‐7. - PubMed
Keith 2001 {published data only}
-
- Keith P, D' Urzo A, Stepner N, et al. Fluticasone/salmeterol combination (FSC) is safe and provides effective long‐term (52 week) control in the management of patients with persistent asthma (PA). European Respiratory Journal 2001; Vol. 18, issue Suppl 33:176s.
Kelsen 1999 {published data only}
-
- Kelsen SG, Church NL, Gillman SA, Lanier BQ, Emmett AH, Rickard KA, et al. Salmeterol added to inhaled corticosteroids therapy is superior to doubling the dose of inhaled corticosteroids: a randomized clinical trial. Journal of Asthma 1999;36(8):703‐15. - PubMed
Kerwin 2001 {published data only}
-
- Kerwin E, Srebro S, Church N, Emmett A, Rickard K, Knobil K. Salmeterol added to low‐dose fluticasone propionate (fp) improves pulmonary function and albuterol use more rapidly than adding montelukast. Annals of Allergy, Asthma & Immunology 2001;86:99.
Ketchell 2002 {published data only}
-
- Ketchell RI, Jensen MW, Spina D, O'Connor BJ. Dose‐related effects of formoterol on airway responsiveness to adenosine 5'‐monophosphate and histamine. European Respiratory Journal 2002;19(4):611‐16. - PubMed
Kidney 1995 {published data only}
-
- Kidney J, Pizzichini MMM, Wong B, Morris MM, Efthimadis A, Dolovich J, et al. Salmeterol compared with beclomethasone and placebo on allergen induced asthmatic and inflammatory responses. European Respiratory Journal 1995; Vol. 8, issue Suppl 19:336s. - PubMed
Kips 2000 {published data only}
-
- Kips JC, O'Connor BJ, Inman MD, Svenson K, Pauwels RA, O'Byrne PM. A long‐term study of the antiinflammatory effect of low‐dose budesonide plus formoterol versus high‐dose budesonide in asthma. American Journal Respiratory Critical Care Medicine 2000;161:996‐1001. - PubMed
Kirby 2000 {published data only}
-
- Kirby S, Falcoz C, Daniel MJ, Milleri S, Squassante L, Ziviani L, et al. Salmeterol and fluticasone propionate given as a combination: lack of systemic pharmacodynamic and pharmacokinetic interactions. European Journal of Clinical Pharmacology 2000;56(11):781‐91. - PubMed
Knobil 2000 {published data only}
-
- Knobil K, Dorinsky P, Yancey S, Emmett A, Rickard K. Salmeterol is superior to montelukast as add‐on therapy to inhaled corticosteroids. European Respiratory Journal 2000; Vol. 16, issue Suppl 31:457s.
Knorr 2001 {published data only}
-
- Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001;108(3):E48. - PubMed
Kraft 2003 {published data only}
-
- Kraft M, Martin RJ, Lazarus SC, Lemanske RF, Szefler SJ. Airway tissue mast cells in persistent asthma: predictor of treatment failure when patients discontinue inhaled corticosteroids. Chest 2003;124(1):42‐50. - PubMed
LaForce 1994 {published data only}
-
- LaForce C, Liddle RF, Yancey SW. Salmeterol response in asthmatic patients using inhaled corticosteroids and in those not using inhaled corticosteroids. Annals of Allergy. 1994; Vol. 72:100.
Lai 1995 {published data only}
-
- Lai CKW, Chan CHS, Ho SS, Hui ACF, Lai KN. Inhaled salmeterol and albuterol in asthmatic patients receiving high‐dose inhaled corticosteroids. Chest 1995;108:36‐40. - PubMed
Lalloo 2000 {published data only}
-
- Lalloo UG, Bantje TA, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Low‐dose Symbicort (budesonide/formoterol) is more effective than double‐dose inhaled corticosteroid in mild asthma. Allergy Clinical Immunology Int 2000; Vol. Suppl 2:122.
Lalloo 2001a {published data only}
-
- Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Symbicort® (budesonide and formoterol in a single inhaler) is more effective than increasing the dose of inhaled corticosteroids in mild asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Lalloo 2001b {published data only}
-
- Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Budesonide and formoterol in a single inhaler is more effective than a higher dose of inhaled corticosteroid in mild‐moderate persistent asthma. European Respiratory Journal 2001;18(Suppl 33):159s.
Lalloo 2001c {published data only}
-
- Lalloo UG, Malolepsky J, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Budesonide and formoterol in a single inhaler controls exacerbations more effectively than a higher dose of inhaled corticosteroids alone, in mild‐moderate persistent asthma. European Respiratory Journal 2001;18(Suppl 33):43s.
Lalloo 2003 {published data only}
-
- Lalloo UG, Malolepszy D, Kozma K, Krofta J, Ankerst B, Johansen NC, et al. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild to moderate asthma. Chest 2003;123(5):1480‐7. - PubMed
Lange 2001 {published data only}
-
- Lange ML, House KW, Scott CA, Shah TP, Akveld MLM. The salmeterol/fluticasone propionate combination 50/100µg bid is effective as initial maintenance therapy in mild and moderate asthmatics. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:176s.
Lazarus 2001 {published data only}
-
- Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, et al. Long‐acting beta2 agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285(20):2583‐93. - PubMed
Lee 2003 {published data only}
Lemanske 2001 {published data only}
-
- Lemanske RF Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285(20):2594‐603. - PubMed
Lenney 1995 {published data only}
-
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins MM. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154:983‐90. - PubMed
Leuppi 2003 {published data only}
-
- Leuppi FD, Salzberg M, Meyer L, Bucher SE, Nief M, Brutsche MH, et al. An individualized, adjustable maintenance regimen of budesonide/formoterol provides effective asthma symptom control at a lower overall dose than fixed dosing. Swiss Medical Weekly 2003;133(21‐2):302‐9. - PubMed
LHSRG 2000 {published data only}
-
- The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1902‐9. - PubMed
Lindqvist 2001 {published data only}
-
- Lindqvist AE, Karjalainen EM, Laitinen LA, Kava T, Altraja A, Pulkkinen M, et al. Salmeterol (SLM), fluticasone propionate (FP) or disodium cromoglycate (DSCG) in the treatment of newly diagnosed asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Lindqvist 2003 {published data only}
-
- Lindqvist A, Karjalainen EM, Laitinen L A, Kava T, Altraja A, Pulkkinen M, et al. Salmeterol resolves airway obstruction but does not possess anti‐eosinophil efficacy in newly diagnosed asthma: a randomized, double‐blind, parallel group biopsy study comparing the effects of salmeterol, fluticasone propionate, and disodium cromoglycate . Journal of Allergy & Clinical Immunology 2003;112(1):23‐8. - PubMed
Lipworth {published data only}
-
- Lipworth BJ. Does genetic polymorphism of b2‐adrenoceptors determine airway sensitivity to regular long‐acting b2‐agonist therapy?. National Research Register (UK). [N0405016375]
Lipworth 1996 {published data only}
-
- Lipworth BJ, Hall IP, Aziz I, Tan KS, Wheatley A. Beta2‐adrenoceptor polymorphism and bronchoprotective sensitivity with regular short‐ and long‐acting beta2‐agonist therapy. Clinical Science 1996;3:253‐9. - PubMed
Lipworth 1998 {published data only}
-
- Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. American Journal of Medicine 1998;104(5):431‐8. - PubMed
Lipworth 1999a {published data only}
-
- Lipworth BJ, Aziz I. A high dose of albuterol does not overcome bronchoprotective subsensitivity in asthmatic subjects receiving regular salmeterol or formoterol. Journal of Allergy & Clinical Immunology 1999;103(1 part 1):88‐92. - PubMed
Lipworth 1999b {published data only}
-
- Lipworth BJ, Hall IP, Aziz I, Tan KS, Wheatley A. Beta2‐adrenoceptor polymorphism and bronchoprotective sensitivity with regular short‐ and long‐acting beta2‐agonist therapy. Clinical Science 96;3:253‐9. - PubMed
Lipworth 2000a {published data only}
-
- Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous glycine‐16 beta(2)‐adrenoceptor polymorphism. Chest 2000;118(2):321‐8. - PubMed
Lipworth 2000b {published data only}
-
- Lipworth BJ, Dempsey OJ, Aziz I, Wilson AM. Effects of adding a leukotriene antagonist or a long‐acting beta(2)‐agonist in asthmatic patients with the glycine‐16 beta(2)‐adrenoceptor genotype. American Journal of Medicine 2000;109(2):114‐21. - PubMed
Lockey 1999 {published data only}
-
- Lockey RF, DuBuske LM, Friedman B, Petrocella V, Cox F, Rickard K. Nocturnal asthma: effect of salmeterol on quality of life and clinical outcomes. Chest 1999;115(3):666‐73. - PubMed
Lowhagen 2002 {published data only}
-
- Lowhagen O, Wever AM, Lusuardi M, Moscato G, Backer WA, Gandola L, at al. The inflammatory marker serum eosinophil cationic protein (ECP) compared with PEF as a tool to decide inhaled corticosteroid dose in asthmatic patients. Respiratory Medicine 2002;96(2):95‐101. - PubMed
Lundback 2002 {published data only}
-
- Lundback B, Ronmark E, Jonsson AC, Larsson LG, Petavy F, James MH. Airway hyperresponsiveness and treatment effectiveness during a one year study of the combination of salmeterol and fluticasone propionate (FP) compared with FP and salmeterol alone in mild to moderate asthma. European Respiratory Society Annual Congress. 2002:P2397.
Lyseng‐Williamson 2003 {published data only}
-
- Lyseng‐Williamson KA, Plosker GL. Inhaled salmeterol/fluticasone propionate combination: a pharmacoeconomic review of its use in the management of asthma. Pharmacoeconomics 2003;21(13):951‐89. - PubMed
Lötvall 2002 {published data only}
-
- Lötvall J, Woude HJ, Palmqvist M, Arvidsson P, Beckman O, Boorsma M, et al. More rapid onset of action of budesonide/formoterol (Symbicort®) than salmeterol/fluticasone (Seretide™). American Journal of Respiratory & Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A567.
Magadle 2001 {published data only}
-
- Magadle R, Berar‐Yanay N, Weiner P. Long‐acting bronchodilators in premenstrual exacerbation of asthma. Respiratory Medicine 2001;95(9):740‐3. - PubMed
Malmqvist‐Granlund 2000 {published data only}
-
- Malmqvist‐Granlund K, Asking L, Lindbald T, Rollwage U, Steckel H. An in vitro comparison of budesonide/formoterol and fluticasone/salmeterol in dry powder inhalers. European Respiratory Journal 2000; Vol. 16, issue Suppl 31:455s.
Malolepszy 2001 {published data only}
-
- Malolepszy J, Boszormenyi Nagy G, Selroos O, Larsson P, Brander R. Safety of formoterol turbuhaler at cumulative dose of 90 mug in patients with acute bronchial obstruction. European Respiratory Journal 2001;18(6):928‐34. - PubMed
Malolepszy 2002 {published data only}
-
- Malolepszy J. Efficacy and tolerability of oral theophylline slow‐release versus inhaled formoterol in moderate asthma poorly controlled on low‐dose steroids. Atemwegs‐ und Lungenkrankheiten 2002;28(2):78‐87.
Martinat 2003 {published data only}
-
- Martinat Y, Desfougeres JL. Fixed‐dose fluticasone‐salmeterol combination: at least effective and better tolerated than open‐dose combinations. Revue De Pneumologie Clinique 2003;59(3):139‐48. - PubMed
Matz 2001 {published data only}
-
- Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low‐dose fluticasone versus higher‐dose fluticasone: an analysis of asthma exacerbations. Journal of Allergy and Clinical Immunology 2001;107(5):783‐9. - PubMed
McCarthy 2000 {published data only}
-
- McCarthy TP, Boone R, Yancey S, Rickard K. Salmeterol compared to montelukast as adjunctive therapy to inhaled corticosteroids. Thorax 2000; Vol. 55, issue Suppl 3:A63.
McCarthy 2001a {published data only}
-
- McCarthy TP, Russell D, Baxter LE, et al. A comparison of the efficacy of salmeterol/fluticasone propionate combination (SF) with beclometasone dipropionate (BDP) delivered via metered dose inhaler (MDI) in patients not well controlled on bronchodilators alone. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:53‐54s.
McCarthy 2001b {published data only}
-
- McCarthy TP, Greening AP, Holgate SK, Whitehead C, Rice L. The efficacy of salmeterol/fluticasone propionate combination (SFC) metered dose inhaler compared with beclomethasone dipropionate (BDP) in patients not well controlled at step 1 of the British guidelines on asthma management (BGAM). Thorax 2001; Vol. 56, issue Suppl 3:iii62.
McCarthy 2002 {published data only}
-
- McCarthy TP, Grening AP, Holgate SK, Whitehead C, Rice L. Salmeterol/fluticasone propionate combination (SFC) is more effective that beclometasone dipropionate (BDP) in patients not well controlled on bronchodilators alone. American Journal of Respiratory & Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A566.
McCarthy 2003 {published data only}
-
- McCarthy TP, Woodcock A A, Pavord I D, Allen DJ, Parker D, Rice L. A comparison of the anti‐inflammatory and clinical effects of salmeterol 25mcg/fluticasone propionate 50mcg combination (SFC 50) with fluticasone propionate (FP) plus montelukast (M) in patients with mild to moderate asthma. American Thoracic Society 99th International Conference. 2003:B036 Poster H89.
Mcivor 1998 {published data only}
-
- Mcivor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1998;158(3):924‐30. - PubMed
Michel 2000 {published data only}
-
- Michel O, Olbrecht J, Moulard D, Sergysels R. Effect of anti‐asthmatic drugs on the response to inhaled endotoxin. Annals of Allergy, Asthma, & Immunology 2000;85(4):305‐10. - PubMed
Midgren 1992 {published data only}
-
- Midgren‐B, Melander‐B, Persson‐G. Formoterol, a new long‐acting beta 2 agonist, inhaled twice daily, in stable asthmatic subjects. Chest 1992;101(4):1019‐22. - PubMed
Miraglia del Giudice 2007 {published data only}
-
- Miraglia del Giudice M, Capristo M, Amelio R, Rocco A, Fusco N, Brunese FP. Combined fluticasone propionate/salmeterol (Diskus) vs fluticasone propionate (Diskus) in the control of persistent asthma in children. American Thoracic Society 99th International Conference. 2003:A117 Poster D78.
-
- Miraglia del Giudice M, Piacentini GL, Capasso M, Capristo C, Maiello N, Boner AL, et al. Formoterol, montelukast, and budesonide in asthmatic children: effect on lung function and exhaled nitric oxide. Respiratory Medicine 2007;101(8):1809‐13. - PubMed
Mitchell 2000 {published data only}
-
- Mitchell C, Jenkins C, Scicchitano R, Rubinfeld A. Adding formoterol is more effective and safer than doubling the dose of inhaled steroids in moderately severe asthma. American Journal of Respiratory & Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A197.
Mitchell 2003 {published data only}
-
- Mitchell C, Jenkins C, Scicchitano R, Rubinfeld A, Kottakis J. Formoterol (Foradil) and medium‐high doses of inhaled corticosteroids are more effective than high doses of corticosteroids in moderate‐to‐severe asthma. Pulmonary Pharmacology & Therapeutics 2003;16(5):229‐306. - PubMed
Murray 1998 {published data only}
-
- Murray JJ, Hagaman DD, Dworski R, Keane B, Sheller JR. Inhibition by salmeterol and beclomethasone of late phase response to segmental antigen challenge in asthmatics. American Journal of Respiratory & Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A872.
Murray 1999 {published data only}
-
- Murray JJ, Church NL, Anderson WH, Bernstein DI, Wenzel SE, Emmett A, et al. Concurrent use of salmeterol with inhaled corticosteroids is more effective than inhaled corticosteroid dose increases. Allergy & Asthma Proceedings 1999;20(3):173‐80. - PubMed
Nathan 1995 {published data only}
-
- Nathan RA, Seltzer JM, Kemp JP, Chervinsky P, Alexander WJ, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma and Immunology 1995;75:243‐8. - PubMed
Nathan 1999a {published data only}
-
- Nathan RA, Pinnas JL, Schwartz HJ, Grossman J, Yancey SW, Emmett AH, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy, Asthma, & Immunology 1999;82:521‐9. - PubMed
Nathan 1999b {published data only}
-
- Nathan R, Woodring A, Baitinger L, Prillaman B, Faris M, House K, et al. The salmeterol/fluticasone propionate Diskus combination decreases the incidence of exacerbations compared to treatment with salmeterol or fluticasone propionate alone. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:848.
Nathan 2001 {published data only}
-
- Nathan RA, Mitchell D, Condemi J, Heller A, Schoaf L, Herrle M, et al. Cardiovascular and hypothalamic‐pituitary‐adrenal axis safety of fluticasone propionate/salmeterol HFA MDI in adolescent and adult patients with asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Nelson 1999 {published data only}
-
- Nelson HS, Berkowitz RB, Tinkelman DA, Emmett AH, Rickard KA, Yancey SW. Lack of sub‐sensitivity to albuterol after treatment with salmeterol in patients with asthma. American Journal of Respiratory and Critical Care Medicine 1999;159(5 part 1):1556‐61. - PubMed
Nelson 2000a {published data only}
-
- Nelson H, Chervinsky P, Greos L, Pelskow W, Baitinger L, Scott C, et al. The salmeterol/fluticasone propionate combination product improves asthma control compared with the individual products in asthmatics treated with prn short‐acting beta2‐agonists alone. American Journal of Respiratory & Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A196.
-
- Nelson HS, Baitinger L, Scott C, House K, Payne E, Shah T. Salmeterol/fluticasone propionate (50/100µg dose) non‐CFC metered dose inhaler is safe and effective in in patients with asthma using short‐acting ß²‐agonists alone. European Respiratory Journal 2000; Vol. 16, issue 31:53s.
-
- Nelson HS, Busse WW, Kerwin E, Church N, Emmett A, Rickard K, et al. Fluticasone propionate/salmeterol combination provides more effective asthma control than low‐dose inhaled corticosteroid plus montelukast. Journal of Allergy and Clinical Immunology 2000;106(6):1088‐95. - PubMed
Nelson 2000b {published data only}
-
- Nelson HS, Chervinsky P, Greos L, Pleskow W, Baitinger L, Scott C, et al. The salmeterol/fluticasone propionate combination product improves asthma control compared with the individual products in asthmatics treated with PRN short‐acting beta2‐agonists alone. American Journal of Respiratory & Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A196.
Nelson 2001 {published data only}
-
- Nelson HS, Nathan RA, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. Medscape General Medicine 2001; Vol. 3, issue 4:3. - PubMed
Newnham 1995 {published data only}
Nielsen 1999 {published data only}
-
- Nielsen LP, Pedersen B, Faurschou P, Madsen F, Wilcke JTR, Dahl R. Salmeterol reduces the need for inhaled corticosteroids in steroid‐dependent asthmatics. Respiratory Medicine 1999;93:863‐8. - PubMed
Nightingale 2002 {published data only}
-
- Nightingale JA, Rogers DF, Barnes PJ. Comparison of the effects of salmeterol and formoterol in patients with severe asthma. Chest 2002;121(5):1401‐6. - PubMed
Nsouli 2001 {published data only}
-
- Nsouli SM, McNutt WJ. The additive effects of montelukast and salmeterol in moderate asthmatics who are uncontrolled on a low dose on inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2001;86:81.
O'Brian 2001 {published data only}
-
- O'Brian J, Carlos‐Palma A, Bogolubov M, Davies P, Payne E. Benefits of fluticasone propionate/salmeterol [fp/s] HFA‐MDI are apparent on the first day of dosing. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Odeback 1998 {published data only}
-
- Odeback P. Is the addition of salmeterol more effective than doubling the dose of budesonide in mild asthma?. American Journal of Respiratory & Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A417.
Olsson 2002 {published data only}
-
- Olsson P, Stallberg B, Ekstrom T, Lindarck N, Jorgensen LA. Adjustable maintenance treatment of asthma with budesonide and formoterol in a single inhaler. European Respiratory Society Annual Congress. 2002:P2451.
Ortega‐Cisnero 1998 {published data only}
-
- Ortega‐Cisnero M, Maldonado‐Alaniz ML, Rosas Vargas MA, Sierra‐Monge JJL. Salmeterol and inhaled beclomethasone versus high dose inhaled beclomethasone in the control of pediatric patients with moderate asthma. Annals of Allergy, Asthma and Immunology 1998;80:131.
Overbeck 2003 {published data only}
-
- Overbeck SE, Mulder PG, Baelemans SM, Hoogsteden HC, Prins JB. Comparison of budesonide plus formoterol with budesonide alone on airway inflammation in mild asthmatics. American Thoracic Society 99th International Conference. 2003:D034 Poster C36.
Ozkaya 1999 {published data only}
-
- Ozkaya O, Turktas I, Cengizlier R. Low dose budesonide plus formoterol versus high dose budesonide in children with bronchial asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999.
Palmer 1992 {published data only}
-
- Palmer JBD, Stuart AM, Shepherd GL, Viskum K. Inhaled salmeterol in the treatment of patients with moderate to severe reversible obstructive airways disease ‐ a 3 month comparison of the efficacy and safety of twice‐daily salmeterol (100 mcg) with salmeterol (50 mcg). Respiratory Medicine 1992;86:409‐17. - PubMed
Palmqvist 2001 {published data only}
-
- Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lotvall J. Onset of bronchodilation of budesonide/formoterol vs salmeterol/fluticasone in single inhalers. Pulmonary Pharmacology & Therapeutics 2001;14(1):29‐34. - PubMed
Paterson 1999 {published data only}
-
- Paterson MC, Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. The effect of combination therapy with salmeterol and montelukast in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:P3490.
Pauwels 1998 {published data only}
-
- Pauwels RA, Yernault JC, Demedts MG, Geusens P. Safety and efficacy of fluticasone and beclomethasone in moderate to severe asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3 pt 1):827‐32. - PubMed
Pearlman 1992 {published data only}
-
- Pearlman DA, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild to moderate asthma. New England Journal of Medicine 1992;327:1420‐5. - PubMed
Pearlman 1994 {published data only}
-
- Pearlman DS, Liddle R. Controlling asthma symptoms: salmeterol compared with salbutamol in large‐scale multicentre studies. European Respiratory Review 1994;4(21):301‐5.
Pearlman 1999a {published data only}
-
- Pearlman DS, Stricker W, Weistein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: a study comparing monotherapy and combination therapy in asthma. Annals of Allergy, Asthma, & Immunology 1999;82:257‐65. - PubMed
Pearlman 1999b {published data only}
-
- Pearlman DS, Stricker W, Weistein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: a study comparing monotherapy and combination therapy in asthma. Annals of Allergy, Asthma, & Immunology 1999;82:257‐65. - PubMed
Pearlman 2001 {published data only}
-
- Pearlman DS, Kent E, Lanz MJ, Peden D, Baitinger L, Herrle M, et al. Fluticasone propionate/salmeterol HFA‐MDI has a rapid onset of effect in asthmatics treated with short or long‐acting beta2‐agonists (ba) or inhaled corticosteroids. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Pearlman 2002 {published data only}
-
- Pearlman DS, White MV, Lieberman AK, Pepsin PJ, Kalberg C, Emmett A, et al. Fluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthma. Annals of Allergy, Asthma and Immunology 2002;88(2):227‐35. - PubMed
Peters 2000 {published data only}
-
- Peters JI, Shelledy DC, Jones AP Jr, Lawson RW, Davis CP, LeGrand TS. A randomized, placebo‐controlled study to evaluate the role of salmeterol in the in‐hospital management of asthma. Chest 2000;118(2):313‐20. - PubMed
Pieters 1999b {published data only}
-
- Pieters WR, Lundback B, Sondhi S, Price MJ. Cost effectiveness analysis of salmeterol/fluticasone propionate 50/500mcg vs fluticasone propionate 500mcg in patients with corticosteroid‐dependent asthma. Pharmacoeconomics 1999;16(Suppl 2):29‐34.
Pieters 2001 {published data only}
-
- Pieters WR, Wilson KK, Smith HCE, Tamminga JJ. Cost‐effectiveness of fluticasone propionate/salmeterol combination product and fluticasone propionate/montelukast in asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Pinnas 1998 {published data only}
-
- Pinnas JL, Schwartz H, Yancey SW, Rickard K. Six month comparison of beclomethasone versus salmeterol or placebo in adults with asthma. American Journal of Respiratory & Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A417.
Pizzichini 1996 {published data only}
-
- Pizzichini MM, Kidney JC, Wong BJ, Morris MM, Efthimiadis A, Dolovich J, et al. Effect of salmeterol compared with beclomethasone on allergen‐induced asthmatic and inflammatory responses. European Respiratory Journal 1996;9(3):449‐55. - PubMed
Pljaskic‐Kamenov 2000 {published data only}
-
- Pljaskic‐Kamenov SS, Filipovic MD, Kamenov BA. Comparison of addition of salmeterol xinafoate to budesonide with budesonide alone on symptoms and quality of life in asthmatic children. European Respiratory Journal 2000; Vol. 16, issue Suppl 31:518s.
Price 2003 {published data only}
-
- Price D, Haughney J, Rosen J P, Morrison K. Switching to Symbicort(r) from beclomethasone dipropionate (BDP) with or without salmeterol significantly improved symptom severity in patients with moderate asthma. American Thoracic Society 99th International Conference. 2003; Vol. D034 Poster C40.
Pujet 1995 {published data only}
-
- Pujet JC, Evano CI. A randomized double‐blind study comparing inhaled beclomethasone with long‐acting theophylline for the first‐line treatment of moderate asthma. Semaine Des Hopitaux 1995;71(27‐8):865‐72.
Pyke 2001 {published data only}
-
- Pyke SD. Synergy with salmeterol and fluticasone propionate after administration from a single inhaler (Seretide(tm)). European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:176s.
Rance 2002 {published data only}
-
- Rance L, Musin L. Asthma management costs in Canada are lower with combination fluticasone propionate/salmeterol (250/50mcg BID) in as single inhaler than with budesonide 800mcg BID plus eformoterol 12 mcg BID via separate inhalers. Chest 2002; San Diego, CA. 2002:S6.
Rickard 1999 {published data only}
-
- Rickard KA, Yancey S, Emmett AH, Kalberg CJ. Salmeterol compared to zafirlukast when added to inhaled corticosteroid therapy in patients with persistent asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:P839.
Rickard 2001 {published data only}
-
- Rickard K, Dorinsky PM, Knobil K, Pepsin P, Akveld ML. The salmeterol/fluticasone propionate combination 50/100µg bid is more effective than oral montelukast 10mg of as a first line therapy in mild and moderate asthmatics. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:262s.
Rijssenbeek‐Nouwens 2002 {published data only}
-
- Rijssenbeek‐Nouwens LH, Oosting AJ, Monchy JG, Bregman I, Postma DS, Bruin‐Weller MS. The effect of anti‐allergic mattress encasings on house dust mite‐induced early‐ and late‐airway reactions in asthmatic patients. A double‐blind, placebo‐controlled study . Clinical & Experimental Allergy 2002;32(1):117‐25. - PubMed
Ringbaek 1996 {published data only}
-
- Ringbaek TJ, Soes‐Petersen U, Christensen M, Iversen ET, Rasmussen FV. Salmeterol improves the control of disease in patients with moderate asthma. A comparative study of inhaled salmeterol 50 mg and salbutamol depot tablets 8 mg, both administered twice daily]. Ugeskrift for Laeger 1996;158(27):3940‐3. - PubMed
Ringdal 1997 {published data only}
-
- Ringdal N, Eliraz A, Pruzinec R, Weber HH, Mulder PG, Akveld M, et al. The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respiratory Medicine 97;3:234‐4. - PubMed
Ringdal 2002 {published data only}
-
- Alonso JF, Badiola C, Kielhorn A. Economic evaluation of salmeterol/fluticasone combination vs budesonide plus formoterol in Spain. European Respiratory Journal 2001;18(Suppl 33):49s.
-
- Chuchalin AG, Chovan L, Ringdal N, Whitehead PJ. Advair™ seretide™ (250/50 mcg bid) shows nocturnal benefit over budesonide 800mu‐g + formoterol 12mu‐g bid in moderate to severe asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
-
- Jenkins C, Wilson J, Rutherford C, Perry AS, Whitehead PJ. Asthma management costs are lower with combination fluticasone/salmeterol (25/50 mcg BD) in a single inhaler than with budesonide (800 mcg BD) plus eformoterol (12 mcg BD) via separate inhalers. Respirology 2002; Vol. 7, issue Suppl:A20. [P23]
-
- Martin AA, Whitehead PJ, McCarthy TP. Asthma costs with salmeterol/fluticasone combination 50/250mcg bd compared to budesonide 800mcg bd plus formoterol 12mcg bd. American Thoracic Society 99th International Conference. 2003:D034 Poster C43.
-
- Ringdal N, Chuchalin A, Chovan L, Tudoric N, Maggi E, Whitehead PJ. Evaluation of different inhaled combination therapies (EDICT): a randomised, double‐blind comparison of Seretide (50/250 microgram bd Diskus vs. formoterol (12 microgram bd) and budesonide (800 microgram bd) given concurrently (both via Turbuhaler) in patients with moderate‐to‐severe asthma. Respiratory Medicine 2002;96(11):851‐61. - PubMed
Ringdal 2003 {published data only}
-
- Ringdal N, Eliraz A, Pruzinec R, Weber HH, Mulder PG, Akveld M, et al. The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respiratory Medicine 97;3:234‐4. - PubMed
Rocca‐Serra 2002 {published data only}
-
- Rocca‐Serra JP, Vicaut E, Lefrancois G, Umile A. Efficacy and tolerability of a new non‐extrafine formulation of beclomethasone HFA‐134a in patients with asthma: comparison with beclomethasone CFC. Clinical Drug Investigation 2002;22(10):653‐65.
Rooklin 2001 {published data only}
-
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The fluticasone propionate/salmeterol HFA MDI is significantly more efficacious in treating asthma than placebo HFA MDI, fluticasone propionate CFC MDI or salmeterol CFC MDI. Journal of Allergy and Clinical Immunology 2001; Vol. 107, issue 2:S100.
Rosenhall 2001a {published data only}
-
- Rosenhall L, Stahl E, Heinig JH, Lindquist A, Leegard J, Bergqvist PBF. Health‐related quality of life and asthma control in patients using Symbicort® (budesonide and formoterol in a single inhaler). Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Rosenhall 2001b {published data only}
-
- Rosenhall L, Heinig JH, Lindqvist A, Leegard J, Bergqvist PB. Budesonide and formoterol in a single inhaler is safe and effective in the treatment of asthma. European Respiratory Journal 2001;18(33):159s.
Rosenhall 2001c {published data only}
-
- Rosenhall L, Stahl E, Heinig JH, Lindqvist A, Leegard J, Bergqvist PB. Health‐related quality of life and asthma control in patients treated with budesonide and formoterol in a single inhaler. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:46s.
Rosenhall 2002 {published data only}
-
- Rosenhall L, Heinig JH, Lindqvist A, Leegaard J, Stahl E, Bergqvist PB. Budesonide/formoterol (Symbicort) is well tolerated and effective in patients with moderate persistent asthma. International Journal of Clinical Practice 2002;56(6):427‐33. - PubMed
Rosenhall 2003 {published data only}
-
- Rosenhall L, Elvstrand A, Tilling B, Vinge I, Jemsby P, Stahl E, et al. One‐year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler ) for the treatment of asthma. Respiratory Medicine 2003;97(6):702‐8. - PubMed
Rosenhall 56 {published data only}
-
- Rosenhall L, Heinig JH, Lindqvist A, Leegard J, Bergqvist PB. Symbicort (budesonide/eformoterol in a single inhaler) is safe and effective in the treatment of asthma. Thorax 56; Vol. Suppl 3:iii 63.
Rosenthal 1999 {published data only}
-
- Rosenthal RR, Busse WW, Kemp JP, Baker JW, Kalberg C, Emmett A, Rickard KA. Effect of long‐term salmeterol therapy compared with as‐needed albuterol use on airway hyperresponsiveness. Chest 1999;116(3):595‐602. - PubMed
Rumbak 1998 {published data only}
-
- Rumbak M, Self T, Kelso T, Eberle L, Abou‐Shala N, Learned S, et al. Moderate to high dose inhaled corticosteroids in adult asthmatics: does salmeterol facilitate step down therapy?. European Respiratory Journal 1998;12(Suppl 29):19s.
Sahn 2002 {published data only}
-
- Sahn S, Yancey S, Reilly D, Edwards L, Rickard K, Dorinsky P. The fluticasone propionate/salmeterol (FSC) combination product 100/50 mcg BID is steroid sparing in patients who require FP250 mcg BID for asthma stability. Chest 2002 (conference); San Diego, CA. 2002.
SAM30007 {unpublished data only}
-
- SAM30007. A multicentre, randomised, double‐blind, controlled, parallel‐group, comparative investigation of the corticosteroid‐saving potential of the combination therapy fluticasone propionate and salmeterol (SERETIDE) compared with fluticasone propionate alone, given to adult asthmatic subjects, when reducing the inhaled corticosteroid dose from an initially high level of 500μg bd. www.ctr.gsk.co.uk 2005 (accessed 5 June 2008).
SAM40004 {published data only}
-
- Beckett P, Hewitt L, Woodcock A, Smith J, Seghal N, Rice L, et al. Improvement in airway hyper‐responsiveness (AHR) and lung function with salmeterol/fluticasone propionate combination (SFC) in persistent asthma [abstract]. American Thoracic Society 99th International Conference. 2003:D034 Poster C27.
-
- SAM40004. A multi‐centre, randomised, double‐blind, placebo‐controlled parallel group study to compare the effect on airway inflammation and remodelling of treatment with salmeterol/fluticasone propionate combination product (50/100μg strength) bd via the Accuhaler inhaler, or fluticasone propionate 100μg bd via the Accuhaler inhaler or placebo via the Accuhaler inhaler for 16 weeks, followed by double‐blind treatment for 52 weeks with the salmeterol/fluticasone propionate combination product (50/100μg strength) bd via the Accuhaler inhaler or fluticasone propionate 100μg bd via the Accuhaler inhaler, in adults with reversible airways obstruction (SIRIAS ‐ Seretide in Inflammation and Remodelling In Asthma Study). http://www.gsk.ctr.co.uk 2006 (accessed 30 April 2008).
Schreurs 1996 {published data only}
-
- Schreurs AJ, Sinninghe Damste HE, Graaff CS, Greefhorst AP. A dose‐response study with formoterol Turbuhaler as maintenance therapy in asthmatic patients. European Respiratory Journal 1996;9:1678‐83. - PubMed
Sears 2003 {published data only}
-
- Sears MR, McIvor A, Becker A, Fitzgearld JM, Boulet LP, Ernsy P, et al. Budesonide/formoterol adjustable maintenance dosing effectively improves asthma symptom severity: a multicentre Canadian study. European Respiratory Journal 2003;22(Suppl 45):P1695.
Serrier 2003 {published data only}
-
- Serrier P, Roche N, Pello JY, Larguier JS, Mezzi K. Asthma control achieved with inhaled corticosteroids and long‐acting beta2‐agonists in a free or fixed combination: results of the ALISE survey. Presse Medicale 2003;32(11):493‐7. - PubMed
Shapiro 2001 {published data only}
-
- Shapiro GG, Mendelson LM, Pearlman DS. Once‐daily budesonide inhalation powder (Pulmicort Turbuhaler) maintains pulmonary function and symptoms of asthmatic children previously receiving inhaled corticosteroids. Annals of Allergy, Asthma, and Immunology 2001;86(6):633‐40. - PubMed
Sheth 2002 {published data only}
-
- Sheth K, Borker R, Emmett A, Rickard K, Dorinsky P. Cost‐effectiveness comparison of salmeterol/fluticasone propionate versus montelukast in the treatment of adults with persistent asthma. Pharmacoeconomics 2002;20(13):909‐18. - PubMed
Shrewsbury 2002 {published data only}
-
- Shrewsbury SB, Pyke S. MIASMA: asthma exacerbation reduction with salmeterol [Letter]. Chest 2002;121(3):1002‐4. - PubMed
Sienra‐Monge 2001 {published data only}
-
- Sienra‐Monge JJL, Rio BE, Alvarez ME, Magana AJ. Comparison of quality of life and pulmonary function on moderate asthmatic children treated with beclomethasone and beclomethasone plus salmeterol. Journal of Allergy and Clinical Immunology 2001; Vol. 107, issue 2:s263.
Simons 1997b {published data only}
-
- Simons FER. A comparison of beclomethasone, salmeterol and placebo in children with asthma. New England Journal of Medicine 1997;337:1659‐65. - PubMed
Sims 2003 {published data only}
Staehr 1995 {published data only}
-
- Staehr P, Vestbo I. Salmeterol improves control in asthmatic patients treated in general practice. A comparative study of salmeterol (Serevent) and salbutamol. Ugeskr‐Laeger 1995;157(1):36‐40. - PubMed
Stahl 2003 {published data only}
-
- Stahl E, Postma DS, Svensson K, Tattersfield AE, Eivindson A, Schreurs A, et al. Formoterol used as needed improves health‐related quality of life in asthmatic patients uncontrolled with inhaled corticosteroids. Respiratory Medicine 2003;97(9):1061‐6. - PubMed
Stallberg 2003 {published data only}
-
- Stallberg B, Olsson P, Jorgensen LA, Lindarck N, Ekstrom T. Budesonide/formoterol adjustable maintenance dosing reduces asthma exacerbations versus fixed dosing. International Journal of Clinical Practice 2003;57(8):656‐61. - PubMed
Stanford 2002 {published data only}
-
- Stanford RH, Borker R, Dorinsky P, Pepsin P, Kalberg C, Emmett A. The costs and efficacy of fluticasone propionate/salmeterol combination versus montelukast in the treatment of adults with persistent asthma. Chest 2002; San Diego, CA. 2002:422.
Stankovic 2000 {published data only}
-
- Stankovic IJ, Djordjevic DV, Pejcic TA, Zivkovic DS, Rancic MR, et al. Formoterol and beclomethasone dipropionate versus higher dose beclomethasone dipropionate in asthma patients. European Respiratory Journal 2000;16(Suppl 31):455s.
Stelmach 2001 {published data only}
-
- Stelmach I, Grzelewski T, Majak P, Majak J, Bobrowska M, Jerzynska J, et al. The effect of triamcinolone, montelukast and formoterol on serum levels of il‐4, IgE and clin.ical parameters in children with asthma. Polski Merkuriusz Lekarski 2001;11(63):247‐51. - PubMed
Stelmach 2002a {published data only}
-
- Stelmach I, Jerzynska J, Majak P, Grzelewski T, Gorski P, Stelmach W, Kuna P. Effect of triamcinolone acetonide, montelukast, nedocromil sodium, formoterol on serum levels of sICAM‐1, sIL‐2R and clinical parameters of asthma in children. Polski Merkuriusz Lekarsk 2002;12(68):99‐103. - PubMed
Stelmach 2002b {published data only}
-
- Stelmach I, Jerzynska J, Kuna P. A randomized, double‐blind trial of the effect of glucocorticoid, antileukotriene and [beta]‐agonist treatment on IL‐10 serum levels in children with asthma. Clinical & Experimental Allergy 2002;32(2):264‐9. - PubMed
Stelmach 2008 {published data only (unpublished sought but not used)}
-
- Stelmach I, Grzelewski T, Jerzynska J, Kuna P. A randomized, double‐blind trial on the effect of treatment with montelukast, budesonide, montelukast with budesonide, formoterol with budesonide on lung function and clinical symptoms in children with asthma [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(Suppl 2):S151.
-
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise‐induced bronchoconstriction in children with asthma. Journal of Allergy and Clinical Immunology 2007; Epub ahead of print (accessed 17 January 2008). - PubMed
Stojkovic‐Andjelkovi 2001 {published data only}
-
- Stojkovic‐Andjelkovic AK, Pajovic DM, Protrka OJ, Ugrinovic BS, Obradovic SM, Pavicevic MD. Effect of combination fluticasone propionate and salmeterol diskhaler in treatment of moderate to severe asthma: comparison of initial high dose, constant medium dose and placebo. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:123s.
Stoloff 2002 {published data only}
-
- Stoloff S, Poinsett‐Holmes K, Dorinsky PM. Combination therapy with inhaled long‐acting beta(2)‐agonists and inhaled corticosteroids: a paradigm shift in asthma management. Pharmacotherapy 2002;22(2):212‐6. - PubMed
Tan 1997 {published data only}
-
- Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between beta 2‐adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet 1997;350(9083):995‐9. - PubMed
Tattersfield 1999 {published data only}
-
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. American Journal of Respiratory & Critical Care Medicine 1999;160(2):594‐9. - PubMed
Tattersfield 2001 {published data only}
-
- Tattersfield AE, Lofdahl CG, Postma DS, Eivindson A, Schreurs AG, Rasidakis A, et al. Comparions of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257‐61. - PubMed
Tolley 2002 {published data only}
-
- Tolley K, Martin A, Rice L, McCarthy TP. Salmeterol/fluticasone propionate combination (SFC) demonstrates improved health outcomes and good cost effectiveness compared with beclometasone dipropionate. American Journal of Respiratory & Critical Care Medicine 2002;165(Suppl 8):A112.
Tonelli 2001 {published data only}
-
- Tonelli M, Giannini D, Franco A, Carnevali S, Bartoli ML, Cianchetti S, et al. Symptoms, induced sputum eosinophil percentages and treatment with salmeterol or fluticasone in mild asthmatics. European Respiratory Journal 2001, issue Suppl 33:336s.
Trautmann 2001 {published data only}
-
- Trautmann M. Treatment with salmeterol/fluticasone propionate (50/250g) inhaler improves lung function, asthma symptoms and quality of life in a large group of patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 2001; Vol. 163, issue 5:A864.
Turner 1998 {published data only}
-
- Turner MO, Johnston PR, Pizzichini E, Pizzichini MM, Hussack PA, Hargreave FE. Anti‐inflammatory effects of salmeterol compared with beclomethasone in eosinophilic mild exacerbations of asthma: a randomized, placebo controlled trial. Canadian Respiratory Journal 1998;5(4):261‐8. - PubMed
Ullman 1990 {published data only}
-
- Ullman A, Hedner J, Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. American Review of Respiratory Disease 1990;142(3):571‐5. - PubMed
Van den Berg 2000 {published data only}
-
- Berg NJ, Ossip MS, Hederos CA, Anttila H, Ribeiro BL, Davies PI. Salmeterol/fluticasone propionate (50/100 ug) in combination in Diskus inhaler (Seretide) is effective and safe in children with asthma. Pediatric Pulmonology 2000;30:97‐105. - PubMed
van der Woude 2001 {published data only}
-
- Woude HJ, Winter TH, Boorsma M, Bergqvist PBF, Aalbers R. More rapid relief of methacholine‐induced bronchoconstriction with budesonide/formoterol than with salmeterol/fluticasone. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:53s.
Van der Woude 2004 {published data only}
-
- Woude HJ, Boorsma M, Bergqvist PBF, Winter TH, Aalbers RH. Budesonide/formoterol in a single inhaler rapidly relieves methacholine‐induced moderate‐to‐severe bronchoconstriction. Pulmonary Pharmacology & Therapeutics 2004;17(2):89‐95. - PubMed
Van Der Woude 2004 {published data only}
-
- Woude HJ, Boorsma M, Bergqvist PB, Winter TH, Aalbers R. Budesonide/formoterol in a single inhaler rapidly relieves methacholine‐induced moderate‐to‐severe bronchoconstriction. Pulmonary Pharmacology & Therapeutics 2004;17(2):89‐95. - PubMed
Van Noord 1999 {published data only}
-
- Schreurs AJ, Noord JA, Mulder PG. Fluticasone propionate (FP) and salmeterol xinafoate (SLM) in patients with mild to moderate asthma. European Respiratory Journal. 1998; Vol. 12, issue Suppl 29:19s, F159.
van Noord 2001 {published data only}
-
- SFCB3023. A multicentre, randomised, double‐blind, double‐dummy, parallel‐group, three‐month comparison of the salmeterol/fluticasone propionate combination product (2x25/250mcg strength) bd via the pressurised metered dose inhaler with salmeterol/fluticasone propionate combination product (1x50/500mcg strength) bd via the Diskus/Accuhaler™ inhaler and with fluticasone propionate (2x 250mcg strength) alone bd via the pressurised metered dose inhaler in adolescents and adults with reversible airways obstruction. http://www.ctr.gsk.co.uk 2004.
-
- Noord JA, Lill H, Carrillo Diaz T, Greefhorst AP, Davies P. Clinical equivalence of a salmeterol/fluticasone propionate combination product delivered via a chlorofluorocarbon‐free metered‐dose inhaler with the Diskus in patients with moderate to severe asthma. Clinical Drug Investigation 2001;21(4):243‐55.
-
- Noord JA, Lill H, Carrillo T, Davies P. Clinical equivalence of salmeterol/fluticasone propionate combination 50/500 bid delivered via metered dose inhaler (MDI) or diskus™ in patients with reversible airways obstruction. American Journal of Respiratory & Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A197.
Van Schayck 2002 {published data only}
-
- Schayck CP, Cloosterman SG, Bijl‐Hofland ID, Hoogen H, Folgering HT, Weel C. Is the increase in bronchial responsiveness or FEV1 shortly after cessation of beta2‐agonists reflecting a real deterioration of the disease in allergic asthmatic patients? A comparison between short‐acting and long‐acting beta2‐agonists. Respiratory Medicine 2002;96(3):155‐62. - PubMed
Vastagh 2003 {published data only}
-
- Vastagh E, Kuna P, Calistru P, Bogdan MA. Efficacy and safety of inhaled budesonide delivered once or twice daily via HFA‐134a in mild to moderate persistent asthma in adult patients. Comparison with budesonide CFC. Respiratory Medicine 2003;97(Suppl D):S20‐S28. - PubMed
Verberne 1997 {published data only}
-
- Verberne AA, Frost C, Roorda RJ, Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. American Journal of Respiratory & Critical Care Medicine 1997;156:688‐95. - PubMed
Vermetten 1999 {published data only}
-
- Vermetten AM, Boermans JM, Luiten WDVF, Mulder PGH, Vermue NA. Comparison of salmeterol with beclomethasone in adult patients with mild persistent asthma who are already on low‐dose inhaled steroids. Journal of Asthma 1999;36(1):97‐106. - PubMed
Vestbo 2000 {published data only}
-
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in patients with mild to moderate chronic obstructive lung disease. Ugeskr‐Laeger 2000;162(4):493‐7. - PubMed
Vickers 2000 {published data only}
-
- Vickers M. Assessment of long‐term efficacy of early introduction of inhaled steroids in asthma. National Health Technology Assessment (NCCHTA).
Vilsvik 2001 {published data only}
-
- Vilsvik J, Ankerst J, Palmqvist M, Persson G, Schaanning J, Schwabe G, et al. Protection against cold air and exercise‐induced bronchoconstriction while on regular treatment with Oxis. Respiratory Medicine 2001;95(6):484‐90. - PubMed
Virchow 2002 {published data only}
-
- Virchow JC. Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest 2002;121(6):2083‐4. - PubMed
Von Berg 1989 {published data only}
-
- von‐Berg A, Berdel D. Formoterol and salbutamol metered aerosols: comparison of a new and an established beta‐2‐agonist for their bronchodilating efficacy in the treatment of childhood bronchial asthma. Pediatric Pulmonology 1989;7(2):89‐93. - PubMed
Von Berg 2003 {published data only}
-
- Berg A, Papageorgiou Saxoni F, Wille S, Carrillo T, Kattamis C, Helms PJ. Efficacy and tolerability of formoterol Turbuhaler in children. International Journal of Clinical Practice 2003;57(10):852‐6. - PubMed
Wallaert 1999 {published data only}
-
- Wallaert B, Brun P, Ostinelli J, Murciano D, Champel F, Blaive B, et al. A comparison of two long‐acting beta‐agonists, oral bambuterol and inhaled salmeterol, in the treatment of moderate to severe asthmatic patients with nocturnal symptoms. The French Bambuterol Study Group. Respiratory Medicine 1999;93(1):33‐8. - PubMed
Wallin 1990 {published data only}
Wallin 1998 {published data only}
-
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Djukanovic R, Wilson S, et al. Effects of formoterol, budesonide and placebo treatment on asthmatic airway inflammation. Annals of Allergy, Asthma, and Immunology 1998; Vol. 80:88.
Wallin 1999 {published data only}
-
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Lundback B, Della‐Cioppa G, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(1):79‐86. - PubMed
Weinberger 2004 {published data only}
-
- Weinberger M. Innovative therapies for asthma: Anti‐IgE ‐ the future?. Paediatric Respiratory Reviews 2004;5(Suppl A):S115‐18. - PubMed
Weinstein 1998 {published data only}
-
- Weinstein SF, Pearlman DS, Bronsky EA, Byrne A, Arledge T, Liddle R, et al. Efficacy of salmeterol xinafoate powder in children with chronic persistent asthma. Annals of Allergy, Asthma and Immunology 1998;81:51‐8. - PubMed
Weinstein 2001 {published data only}
-
- Weinstein SF, Pearlman DS, Condemi JJ, Herrle MR, Scott CA, Payne JE, et al. Superior efficacy of the fluticasone propionate/salmeterol (88/42mcg) HFA‐MDI combination product versus the individual components in asthmatics previously treated with either short‐ or long‐acting beta2‐agonists or inhaled corticosteroids. Journal of Allergy and Clinical Immunology 2001;107(2):S102.
Wempe 1992 {published data only}
-
- Wempe JB, Tammeling EP, Koeter GH, Hakansson L, Venge P, Postma DS. Blood eosinophil numbers and activity during 24 hours: effects of treatment with budesonide and bambuterol. Journal of Allergy and Clinical Immunology 1992;90(5):757‐65. - PubMed
White 2001 {published data only}
-
- White M, Scott C, Herrle MR, Pearlman D, Payne E, House K, et al. Salmeterol/fluticasone propionate (42/88mcg) HFA‐MDI improves asthma control in asthmatics previously treated with short‐ or long‐acting beta2‐agonists or inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2001; Vol. 86:81.
Wilcke 1998 {published data only}
-
- Wilcke JT, Iversen ET, Kok‐Jensen A. Effect of salmeterol is independent of previously inhaled salbutamol: a clinical controlled study. Lung 1998;176(2):133‐9. - PubMed
Wilding 1997 {published data only}
Wilson 1999 {published data only}
-
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of salmeterol and montelukast as second‐line therapy in asthmatic patients not controlled on inhaled corticosteroids. Thorax 1999; Vol. 54, issue Suppl 3:A66, P189.
Wilson 2000 {published data only}
-
- Wilson SJ, Ward JA, Djukanovic R, Wallin A, Sue‐Chu M, Sandstrom T, et al. Effects of high and low dose inhaled fluticasone propionate (FP) compared to low dose FP combined with salmeterol (SAL) on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 2000; Vol. 161, issue Suppl 3:A196.
Wilson 2001a {published data only}
-
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second‐line therapy for asthma not controlled with inhaled corticosteroids. Chest 2001;119(4):1021‐6. - PubMed
Wilson 2001b {published data only}
-
- Wilson SJ, Wallin A, Della‐Cioppa G, Sandstrom T, Holgate ST. Effects of budesonide and formoterol on NF‐kappaB, adhesion molecules, and cytokines in asthma. American Journal of Respiratory & Critical Care Medicine 2001;164(6):1047‐52. - PubMed
Wong 1992 {published data only}
-
- Wong BJ, Dolovich J, Ramsdale EH, O'Byrne P, Gontovnick L, Denburg JA, et al. Formoterol compared with beclomethasone and placebo on allergen‐induced asthmatic responses. American Review of Respiratory Disease 1992;146(5 pt 1):1156‐60. - PubMed
Woolcock 1995 {published data only}
-
- Woolcock A. Continuing patient care with metered‐dose inhalers. Journal of Aerosol Medicine: Deposition, Clearance, and Effects in the Lung 1995;8(Suppl 2):s5‐s10. - PubMed
Woolcock 1996 {published data only}
-
- Woolcock A, Lunback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling the dose of inhaled steroids. American Journal of Respiratory & Critical Care Medicine 1996;153:1481‐8. - PubMed
Yates 1995 {published data only}
-
- Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma: effect on bronchial responsiveness during and after treatment. American Journal of Respiratory & Critical Care Medicine 1995;152(41):1170‐4. - PubMed
Yates 1996 {published data only}
-
- Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long‐acting inhaled beta 2‐agonist. American Journal of Respiratory & Critical Care Medicine 1996;154(6 pt 1):1603‐7. - PubMed
Youngchaiyud 1995 {published data only}
-
- Youngchaiyud P, Permpikul C, Suthamsmai T, Wong E. A double‐blind comparison of inhaled budesonide, long‐acting theophylline, and their combination in treatment of nocturnal asthma. Allergy 1995;50:28‐33. - PubMed
Yurdakul 2002 {published data only}
-
- Yurdakul AS, Calisir HC, Tunctan B, Ogretensoy M. Comparison of second controller medications in addition to inhaled corticosteroid in patients with moderate asthma. Respiratory Medicine 2002;96(5):322‐9. - PubMed
Zarkovic 1998 {published data only}
-
- Zarkovic J, Gotz MH, Holgate ST, Taak NK. Effect of long‐term regular salmeterol treatment in children with moderate asthma. Clinical Drug Investigation 1998;15(3):169‐75.
References to studies awaiting assessment
Bateman 2001 {published data only}
-
- Bateman ED, Beasley R, Silins V, Bogolubov M. Comparison of salmeterol/fluticasone propionate combination 50/100 bid delivered via metered dose inhaler or diskus in patients with reversible airways obstruction. American Journal of Respiratory & Critical Care Medicine 2000;161(Suppl 3):A197.
-
- Bateman ED, Silins V, Bogolubov M. Clinical equivalence of salmeterol/fluticasone propionate in combination (50/100 mcg twice daily) when administered via a chlorofluorocarbon‐free metered dose inhaler or dry powder inhaler to patients with mild‐to‐moderate asthma. Respiratory Medicine 2001;95(2):136‐46. - PubMed
-
- SFCB3022. A multicentre, randomised, double‐blind, double‐dummy, parallel‐group, three‐month comparison of the salmeterol/fluticasone propionate combination product (2x25/50mcg strength) bd via the pressurised metered dose inhaler with salmeterol/fluticasone propionate combination product (1x50/100mcg strength) bd via the Diskus/Accuhaler™ inhaler and with fluticasone propionate (2x 50mcg strength) alone bd via the pressurised metered dose inhaler in adolescents and adults with reversible airways obstruction. www.ctr.gsk.co.uk 2004 (accessed 12 June 2008).
Yancey 1997 {published data only}
-
- Yancey SW, Rickard KA, Emmett A, Cox F. The response to salmeterol or theophylline in asthmatics either receiving or not‐receiving inhaled corticosteroids. Annals of Allergy, Asthma and Immunology 1997; Vol. 78:110.
References to ongoing studies
Aziz {published data only}
-
- Aziz I. Comparison of anti inflammatory effect of once daily inhaled formoterol and once daily inhaled budesonide on inflammatory markers in asthmatic patients. Clinical Pharmacology, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, Scotland, UK.
Barnes a {published data only}
-
- Barnes NC. A randomized parallel 3 arm study to assess asthma control, lung function, costs and quality of life in patients with asthma treated with formoterol twice daily and either budesonide once or twice daily from a novel multidisc dry powder inhaler. Respiratory Medicine, London Chest Hospital, Bonner Road, London, E2 9JX, United Kingdom.
Barnes b {published data only}
-
- Barnes PJ. Effect of low dose formoterol and budesonide on exhaled breath in asthma. Royal Brompton and Harefield NHS Trust.
Bush {published data only}
-
- Bush A. Efficacy and safety of budesonide/formoterol Turbuhaler , compared to budesonide turbuhaler metered dose, in steroid using asthmatic adolescent patients, double blind, double dummy parallel group, Phase III, multicentre study. Department of Paediatrics, Imperial College School of Medicine, Brompton Campus, Dovehouse Street, London, SW3 6LY, United Kingdom). Royal Brompton and Harefield NHS Trust.
Currie {published data only}
-
- Currie GP. Are there additional anti‐inflammatory effects of leukotriene receptor antagonists in persistent asthmatics receiving inhaled steroid alone and combined inhaled steroid/long acting B2‐agonists?. Academic Publications.
Goves {published data only}
-
- Goves JR. Eformoterol in the management of mild asthma ‐ eformoterol Turbohaler R with budesonide Turbohaler R . Department of Paediatrics, Level 4 John Radcliffe Hospital, Headley Way, Headington, Oxford, OX3 9DU.
Hill {published data only}
-
- Hill J. The ASSURE Study ‐ the effectiveness and safety of an individualised Symbicort Turbohaler maintenance dosing regimen (Symbicort Asthma Control Plan) versus Symbicort Turbohaler given as standard regular twice daily therapy. Northern General Hospital NHS Trust.
Kharitonov {published data only}
-
- Kharitonov SA. Effect of inhaled corticosteroids (budesonide 100 micrograms/400 micrograms vs placebo) and formoterol on localisation of glucocorticoid receptor and exhaled markers in asthma. Royal Brompton and Harefield NHS Trust.
Lipworth 2001 {unpublished data only}
-
- Lipworth BJ. Relative lung bioavailability with fluticasone via dry powder inhaler versus: (a) fluticasone/salmeterol combination vis dry powder inhaler; (b) fluticasone/salmeterol combination via HFA‐pMDI plus spacer, in patients with moderate persistent asthma. Academic Publications 2001.
Millar {published data only}
-
- Millar AB. A randomised double‐blind multi‐centre study to evaluate the effect of adding alternative agents to inhaled steroids in adult asthmatics. Lung Research Unit, Southmead Hospital, Southmead Road, Westbury‐on‐Trym, Bristol, BS10 5NB, UK.
Ruggins {published data only}
-
- Ruggins N. Pragmatic trial of add‐on therapy in paediatric asthma. Southern Derbyshire Acute Hospitals NHS Trust, Consultant Paediatrician, Children's Hospital, Uttoxeter Road, Derby, DE22 3NE, England 2003.
Thomson {published data only}
-
- Thomson NC. Bundesonide/formoterol fixed combination in adults with asthma. Respiratory Medicine, Gartnavel General Hospital, Great Western Road, Glasgow, G12 0YN, Scotland.
Young {published data only}
-
- Young KM. Budesonide/formoterol fixed combination in adults with asthma. Cambridge Consortium ‐ Addenbrookes.
Additional references
Adams 2008
-
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] - DOI
Bateman 2008
-
- Bateman E, Nelson H, Bousquet J, Kral K, Sutton L, Ortega H, et al. Meta‐analysis: effects of adding salmeterol to inhaled corticosteroids on serious asthma‐related events. www.annals.org 2008 (accessed 5 June 2008); Vol. 149, issue 1:Epub. - PubMed
Bisgaard 2003
-
- Bisgaard H. Effect of long acting beta 2 agonists on exacerbation rates of asthma in children. Pediatric Pulmonology 2003;36:391‐8. - PubMed
BTS 2007
-
- British Thoracic Society. British Guideline on the Management of Asthma. www.brit‐thoracic.org.uk 2007.
Canadian Paediatic Asthma Guideline 2005
Cates 2002
Cates 2008a
Cates 2008b
Cates 2009a
Cates 2009b
Cochrane Handbook
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration. .Available from www.cochrane‐handbook.org.
D'Alonzo 1997
-
- D'Alonzo GE, Tolep KA. Salmeterol in the treatment of chronic asthma. American Family Physician 1997;56(2):558‐62. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Smith GD, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Publishing, 2001:285‐312.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Ducharme 2006
-
- Ducharme FM, Lasserson TJ, Cates CJ. Long‐acting beta2‐agonists versus anti‐leukotrienes as add‐on therapy to inhaled corticosteroids for chronic asthma. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003137.pub3] - PubMed
Ducharme 2010
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD005533.pub2] - DOI
Egger 1997
Ernst 2006
-
- Ernst P, McIvor A, Ducharme FM, Boulet L‐P, FitzGerald M, Chapman K, et al. Long‐acting inhaled beta‐agonist bronchodilators are safe and effective in conjunction with inhaled corticosteroids. Annals of Internal Medicine 2006;145(9):693‐4. - PubMed
Gibson 2005
GINA 2007
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (NIH publication). http://www.ginasthma.com 2007.
Gleser 1996
-
- Gleser LJ, Olkin I. Models for estimating the number of unpublished studies. Statistics in Medicine 1996;15:2493‐507. - PubMed
Greenland 1985
-
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. - PubMed
Higgins 2003
Manning 2008
NAC 2006
-
- National Asthma Council. Australia. Asthma Management Handbook (http://www.nationalasthma.org.au/publications/amh/amhcont.htm). 5th Edition. Melbourne: National Asthma Campaign, 2006.
NAEPP 2007
-
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR 3) Guidelines for the Diagnosis and Management of Asthma. In: NIH Publication. Bethesda, MD: National Heart, Lung and Blood Institute (available at: http://www.nhlbi.nih.gov/guidelines/asthma/index.htm). Bethesda, MD: National Heart, Lung and Blood Institute, 2007.
Nelson 1995
-
- Nelson HS. Beta‐adrenergic bronchodilators. New England Journal of Medicine 1995;333(8):499‐506. - PubMed
Nelson 2003
-
- Nelson HS, Chapman KR, Pyke SD, Johnson M, Pritchard JN. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. Journal of Allergy and Clinical Immunology 2003;112(1):29‐36. - PubMed
Ni Chroinin 2009a
-
- Ni Chroinin M, Greenstone I, Lasserson TJ, Ducharme FM. Addition of inhaled long acting beta2‐agonists to inhaled steroids as first line therapy for persistent asthma in steroid naive adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [Art. No.: CD005307. DOI: 10.1002/14651858.CD005307.pub2] - PMC - PubMed
Ni Chroinin 2009b
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Salpeter 2006
-
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting ß‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(12):904‐12. - PubMed
SMART
-
- FDA. Smart study site. www.fda.gov/bbs/topics/ANSWERS/2003/ANS01192.html (accessed 18 May 2009).
Storms 2003
-
- Storms W. Clinical trials: are these your patients?. Journal of Allergy & Clinical Immunology 2003;5(Suppl):S107‐S111. - PubMed
Walters 2007
-
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] - DOI - PMC - PubMed
Warner 1998
-
- Warner JO, Naspitz CK. Third International Pediatric Consensus statement on the management of childhood asthma. International Pediatric Asthma Consensus Group. Pediatric Pulmonology 1998;25(1):1‐17. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

