Alkyne elementometalation-Pd-catalyzed cross-coupling. Toward synthesis of all conceivable types of acyclic alkenes in high yields, efficiently, selectively, economically, and safely: "green" way
- PMID: 20465291
- PMCID: PMC2933819
- DOI: 10.1021/jo1003218
Alkyne elementometalation-Pd-catalyzed cross-coupling. Toward synthesis of all conceivable types of acyclic alkenes in high yields, efficiently, selectively, economically, and safely: "green" way
Abstract
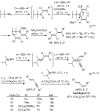
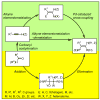
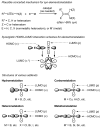
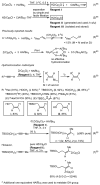
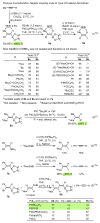
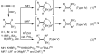
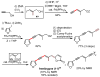
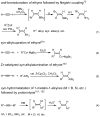
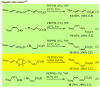
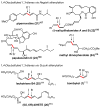
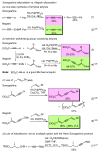
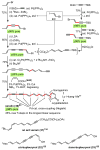
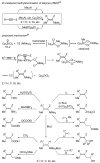
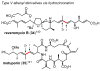
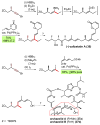
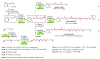
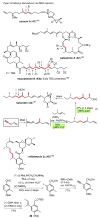
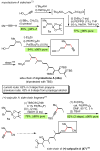
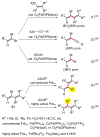
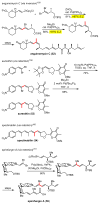
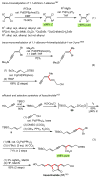
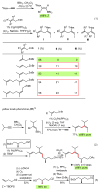
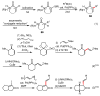
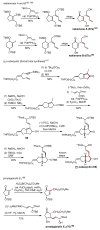
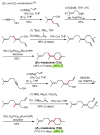
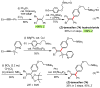
Palladium-catalyzed cross-coupling reactions, especially those involving Zn, Al, Zr (Negishi coupling), and B (Suzuki coupling), collectively have brought about "revolutionary" changes in organic synthesis. Thus, two regio- and stereodefined carbon groups generated as R(1)M (M = Zn, Al, B, Cu, Zr, etc.) and R(2)X (X = I, Br, OTs, etc.) may now be cross-coupled to give R(1)-R(2) with essentially full retention of all structural features. For alkene syntheses, alkyne elementometalation reactions including hydrometalation (B, Al, Zr, etc.), carbometalation (Cu, Al-Zr, etc.), and haloboration (BX(3) where X is Cl, Br, and I) have proven to be critically important. Some representative examples of highly efficient and selective (>or=98%) syntheses of di-, tri-, and oligoenes containing regio- and stereodefined di- and trisubstituted alkenes of all conceivable types will be discussed with emphasis on those of natural products. Some interesting but undesirable cases involving loss of the initial structural identities of the alkenyl groups are attributable to the formation of allylpalladium species, which must be either tamed or avoided. Some such examples involving the synthesis of 1,3-, 1,4-, and 1,5-dienes will also be discussed.
Figures





















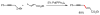




References
-
-
For alkylation of aryl and alkenyl halides with alkyllithiums via rapid halogen-lithium exchange, see: Merrill RE, Negishi E. J Org Chem. 1974;39:3452–3453.Linstrumelle G, Michelot D. J C S Chem Comm. 1975:561–562.
-
-
-
For listing of the Negishi, Suzuki, and Stille coupling reactions as the three representative Pd-catalyzed cross-coupling reactions, see O'Neal MJ, Smith A, Heckelman PE, editors. The Merck Index. 13th. Merck & Co., Inc.; Whitehouse Station, NJ: 2001. ONR-73, ONR-100, ONR-102.Kürti L, Czakó B. Strategic Applications of Named Reactions in Organic Synthesis. Elsevier Academic Press; Burlington, MA: 2005. p. 758.
-
-
-
For early and recent overviews of the Negishi coupling, see Negishi E. Acc Chem Res. 1982;15:340.Negishi E. Bull Chem Soc Jpn. 2007;80:233–257.
-
-
- Miyaura N, Suzuki A. Chem Rev. 1995;95:2457–2483.
- Suzuki A, Brown HC. Organic Syntheses via Boranes Vol 3: Suzuki Coupling. Aldrich Chemical Co., Inc.; Milwaukee, WI: 2003. p. 314.
- Miyaura N, Yamada A, Suzuki A. Tetrahedron Lett. 1979;20:3437–3440.
- Miyaura N, Suzuki A. J Chem Soc Chem Comm. 1979:866–867.
- Yanagi T, Oh-e T, Miyaura N, Suzuki A. Bull Chem Soc Jpn. 1989;62:3892–3895.
-
-
(a) For a review, see: Farina V, Krishnamurthy V, Scott WJ. Org React. 1997;50:1–652. (b) For a seminal contribution by Kosugi, see: Kosugi M, Sasazawa K, Shimizu Y, Migita T. Chem Lett. 1977:301–302. (c) Milstein D, Stille JK. J Am Chem Soc. 1979;101:4981–4991. (d) Milstein D, Stille JK. J Am Chem Soc. 1979;101:4992–4998.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

