Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential
- PMID: 20465527
- PMCID: PMC3035701
- DOI: 10.2217/rme.10.30
Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential
Abstract
Background: Potent stem/progenitor cells have been isolated from normal human dental pulps termed dental pulp stem cells (DPSCs). However, it is unknown whether these cells exist in inflamed pulps (IPs).
Aims: To determine whether DPSCs can be identified and isolated from IPs; and if they can be successfully cultured, whether they retain tissue regeneration potential in vivo.
Materials & methods: DPSCs from freshly collected normal pulps (NPs) and IPs were characterized in vitro and their tissue regeneration potential tested using an in vivo study model.
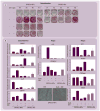
Results: The immunohistochemical analysis showed that IPs expressed higher levels of mesenchymal stem cell markers STRO-1, CD90, CD105 and CD146 compared with NPs (p < 0.05). Flow cytometry analysis showed that DPSCs from both NPs and IPs expressed moderate to high levels of CD146, stage-specific embryonic antigen-4, CD73 and CD166. Total population doubling of DPSCs-IPs (44.6 + or - 2.9) was lower than that of DPSCs-NPs (58.9 + or - 2.5) (p < 0.05), and DPSCs-IPs appeared to have a decreased osteo/dentinogenic potential compared with DPSCs-NPs based on the mineral deposition in cultures. Nonetheless, DPSCs-IPs formed pulp/dentin complexes similar to DPSCs-NPs when transplanted into immunocompromised mice.
Conclusion: DPSCs-IPs can be isolated and their mesenchymal stem cell marker profiles are similar to those from NPs. Although some stem cell properties of DPSCs-IPs were altered, cells from some samples remained potent in tissue regeneration in vivo.
Figures




References
-
- Reffelmann T, Könemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–308. - PubMed
-
- Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281–4298. - PubMed
-
- Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24:347–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
