Ligation of CD11c during vaccination promotes germinal centre induction and robust humoral responses without adjuvant
- PMID: 20465572
- PMCID: PMC2966766
- DOI: 10.1111/j.1365-2567.2010.03285.x
Ligation of CD11c during vaccination promotes germinal centre induction and robust humoral responses without adjuvant
Abstract
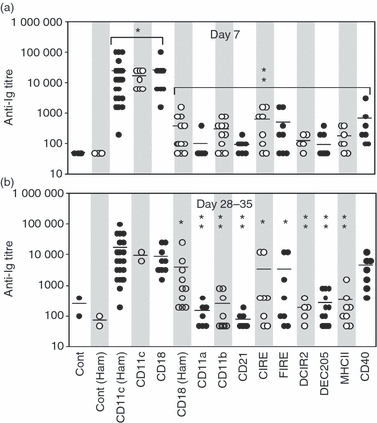
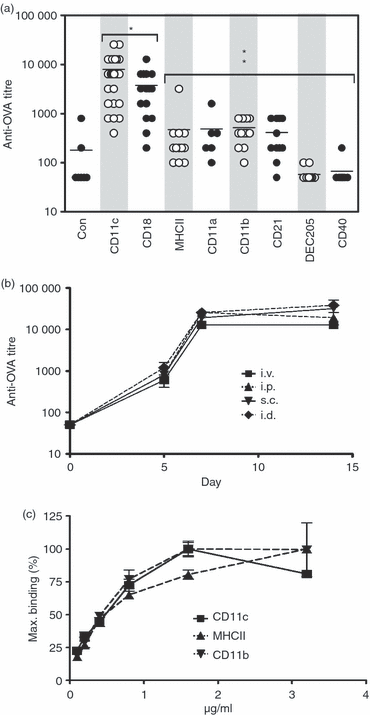
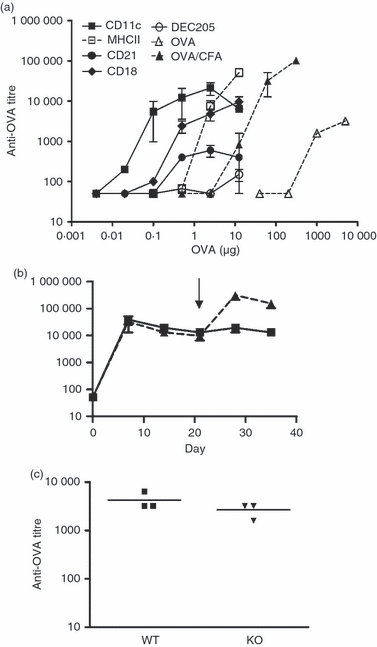
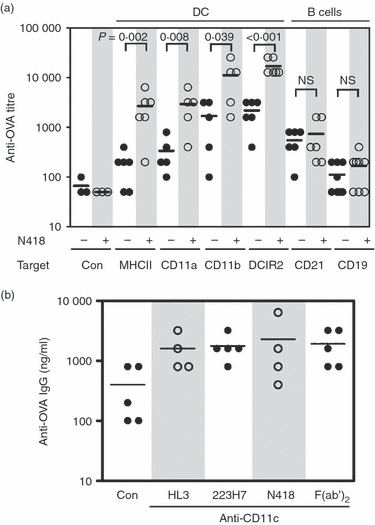
In this study, we investigated the mouse dendritic cell (DC) receptor, complement receptor 4 (CR4; CD11c/CD18), as an immunotarget for triggering humoral immunity. Comparison of antibody titres generated against a panel of 13 anti-antigen-presenting cell receptor monoclonal antibodies, with or without conjugated ovalbumin (OVA), revealed uniquely rapid and robust responses following CR4 targeting, with antibody titres approaching 1 : 100 000 7 days after a single dose of antigen. Furthermore, using just 100 ng OVA conjugated to anti-CD11c Fab', we generated anti-OVA titres greater than those produced by a 100-fold higher dose of OVA in complete Freund's adjuvant at day 28. These anti-OVA antibody titres were sustained and could be boosted further with targeted OVA on day 21. Investigations to explain this vaccine potency showed that, in addition to targeting splenic DC, anti-CDl1c antibodies delivered a powerful adjuvant effect and could boost humoral immunity against OVA even when the OVA was targeted to other molecules on DC, such as major histocompatibility complex class II, CD11a and CD11b. However, interestingly, this adjuvant effect was lost if OVA was targeted to other cells such as B cells via CD21 or CD19. The adjuvant effect was mediated through a marked enhancement of both germinal centre and extrafollicular plasma cell formation in responding spleens. These results demonstrate that anti-CD11c monoclonal antibody can both target antigen and act as a powerful adjuvant for rapid and sustained antibody responses. They also point to an interesting role for CR4 on DC in triggering B cells during humoral immunity.
Figures
Similar articles
-
Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance.J Exp Med. 2002 Dec 16;196(12):1627-38. doi: 10.1084/jem.20021598. J Exp Med. 2002. PMID: 12486105 Free PMC article.
-
The STING activator c-di-AMP exerts superior adjuvant properties than the formulation poly(I:C)/CpG after subcutaneous vaccination with soluble protein antigen or DEC-205-mediated antigen targeting to dendritic cells.Vaccine. 2019 Aug 14;37(35):4963-4974. doi: 10.1016/j.vaccine.2019.07.019. Epub 2019 Jul 15. Vaccine. 2019. PMID: 31320219
-
Chemical Conjugation of a Purified DEC-205-Directed Antibody with Full-Length Protein for Targeting Mouse Dendritic Cells In Vitro and In Vivo.J Vis Exp. 2021 Feb 5;(168). doi: 10.3791/62018. J Vis Exp. 2021. PMID: 33616089
-
Impact of antigen-adjuvant associations on antigen uptake and antigen-specific humoral immunity in mice following intramuscular injection.Biomed Pharmacother. 2019 Oct;118:109373. doi: 10.1016/j.biopha.2019.109373. Epub 2019 Aug 29. Biomed Pharmacother. 2019. PMID: 31545268
-
Multifaceted, unique role of CD11c in leukocyte biology.Front Immunol. 2025 Mar 4;16:1556992. doi: 10.3389/fimmu.2025.1556992. eCollection 2025. Front Immunol. 2025. PMID: 40103815 Free PMC article. Review.
Cited by
-
Targeting Conventional Dendritic Cells to Fine-Tune Antibody Responses.Front Immunol. 2019 Jul 4;10:1529. doi: 10.3389/fimmu.2019.01529. eCollection 2019. Front Immunol. 2019. PMID: 31333661 Free PMC article. Review.
-
CD11c controls herpes simplex virus 1 responses to limit virus replication during primary infection.J Virol. 2011 Oct;85(19):9945-55. doi: 10.1128/JVI.05208-11. Epub 2011 Jul 20. J Virol. 2011. PMID: 21775452 Free PMC article.
-
Enhanced germinal center reaction by targeting vaccine antigen to major histocompatibility complex class II molecules.NPJ Vaccines. 2019 Feb 11;4:9. doi: 10.1038/s41541-019-0101-0. eCollection 2019. NPJ Vaccines. 2019. PMID: 30775000 Free PMC article.
-
Generation of Immunity against Pathogens via Single-Domain Antibody-Antigen Constructs.J Immunol. 2016 Dec 15;197(12):4838-4847. doi: 10.4049/jimmunol.1600692. Epub 2016 Nov 7. J Immunol. 2016. PMID: 27821668 Free PMC article.
-
CD11c-specific bio-nanocapsule enhances vaccine immunogenicity by targeting immune cells.J Nanobiotechnology. 2018 Aug 4;16(1):59. doi: 10.1186/s12951-018-0386-6. J Nanobiotechnology. 2018. PMID: 30077180 Free PMC article.
References
-
- Tarlinton DM, Smith KG. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol Today. 2000;21:436–41. - PubMed
-
- Brink R, Phan TG, Paus D, Chan TD. Visualizing the effects of antigen affinity on T-dependent B-cell differentiation. Immunol Cell Biol. 2008;86:31–9. - PubMed
-
- Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

