High content cellular immune profiling reveals differences between rhesus monkeys and men
- PMID: 20465573
- PMCID: PMC2966765
- DOI: 10.1111/j.1365-2567.2010.03284.x
High content cellular immune profiling reveals differences between rhesus monkeys and men
Abstract
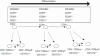
A better understanding of similarities and differences in the composition of the cellular immune system in non-human primates (NHPs) compared with human subjects will improve the interpretation of preclinical studies. It will also aid in addressing the usefulness of NHPs as subjects for studying chronic diseases, vaccine development and immune reconstitution. We employed high content colour flow cytometry and analysed simultaneously the expression of CD3, CD4, CD8alpha, CD8beta, CD16/CD56, CD45RA, CCR7, CD27, CD28, CD107a and the interleukin-7 receptor alpha-chain (IL-7Ralpha) in peripheral blood mononuclear cells (PBMCs) of 27 rhesus macaques and 16 healthy human subjects. Regulatory T cells (Tregs) were identified using anti-CD3, -CD4, -CD25, -FoxP3, and -IL-7Ralpha monoclonal antibodies. Responsiveness to IL-7 was gauged in a signal transducer and activation of transcription 5 (STAT-5) phosphorylation assay. Human and NHP PBMCs showed a similar T-cell composition pattern with some remarkable differences. Similarities: human and NHP CD4(+) and CD8(+) cells showed a similar STAT-5 phosphorylation pattern in response to IL-7. Multicolour flow cytometric analysis identified a CD4(+) CD8alphaalpha(+) CD8alphabeta(+) T-cell population in NHPs as well as in human subjects that expressed the degranulation marker CD107a and may represent a unique CD4(+) T-cell subset endowed with cytotoxic capacity. Differences: we identified in PBMCs from NHPs a higher proportion (5.16% in CD3(+) T cells) of CD8alphaalpha(+) T cells when compared with human donors (1.22% in CD3(+) T cells). NHP CD8alphaalpha(+) T cells produced tumour necrosis factor-alpha / interferon-gamma (TNF-alpha/IFN-gamma) or TNF-alpha, whereas human CD8alphaalpha(+) T cells produced simultaneously TNF-alpha/IFN-gamma and IL-2. A minor percentage of human CD8(+) T cells expressed CD25(bright) and FoxP3 (0.01%). In contrast, 0.07% of NHP CD8(+) T cells exhibited the CD25(bright) FoxP3(+) phenotype. PBMCs from NHPs showed less IL-7Ralpha-positive events in all T-cell subsets including CD4(+) Tregs (median 5%) as compared with human (median 12%). The data visualize commonalities and differences in immune cell subsets in humans and NHPs, most of them in long-lived memory cells and cells with suppressive functions. This provides a matrix to assess future efforts to study diseases and vaccines in NHPs.
Figures


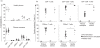



Similar articles
-
Foxp3(high) and Foxp3(low) Treg cells differentially correlate with T helper 1 and natural killer cells in peripheral blood.Hum Immunol. 2011 Aug;72(8):621-6. doi: 10.1016/j.humimm.2011.03.013. Epub 2011 May 11. Hum Immunol. 2011. PMID: 21600259
-
Elevated T regulatory cells in long-term stable transplant tolerance in rhesus macaques induced by anti-CD3 immunotoxin and deoxyspergualin.J Immunol. 2005 Dec 15;175(12):8060-8. doi: 10.4049/jimmunol.175.12.8060. J Immunol. 2005. Retraction in: J Immunol. 2006 Aug 1;177(3):2023. doi: 10.4049/jimmunol.177.3.2023. PMID: 16339543 Retracted.
-
Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques.J Immunol Methods. 2007 Aug 31;325(1-2):20-34. doi: 10.1016/j.jim.2007.05.011. Epub 2007 Jun 12. J Immunol Methods. 2007. PMID: 17628586 Free PMC article.
-
Porcine T lymphocytes and NK cells--an update.Dev Comp Immunol. 2009 Mar;33(3):310-20. doi: 10.1016/j.dci.2008.06.003. Epub 2008 Jul 2. Dev Comp Immunol. 2009. PMID: 18601948 Review.
-
The role of interleukin-2 during homeostasis and activation of the immune system.Nat Rev Immunol. 2012 Feb 17;12(3):180-90. doi: 10.1038/nri3156. Nat Rev Immunol. 2012. PMID: 22343569 Review.
Cited by
-
Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates.Vaccines (Basel). 2024 Sep 26;12(10):1099. doi: 10.3390/vaccines12101099. Vaccines (Basel). 2024. PMID: 39460266 Free PMC article.
-
Pathogenicity of Ebola and Marburg Viruses Is Associated With Differential Activation of the Myeloid Compartment in Humanized Triple Knockout-Bone Marrow, Liver, and Thymus Mice.J Infect Dis. 2018 Nov 22;218(suppl_5):S409-S417. doi: 10.1093/infdis/jiy269. J Infect Dis. 2018. PMID: 30085162 Free PMC article.
-
Contemporary Animal Models For Human Gene Therapy Applications.Curr Gene Ther. 2015;15(6):531-40. doi: 10.2174/1566523215666150929110424. Curr Gene Ther. 2015. PMID: 26415576 Free PMC article. Review.
-
Pre-existing immunity does not impair the engraftment of CRISPR-Cas9-edited cells in rhesus macaques conditioned with busulfan or radiation.Mol Ther Methods Clin Dev. 2023 Apr 20;29:483-493. doi: 10.1016/j.omtm.2023.04.004. eCollection 2023 Jun 8. Mol Ther Methods Clin Dev. 2023. PMID: 37273902 Free PMC article.
-
Induction of experimental autoimmune encephalomyelitis with recombinant human myelin oligodendrocyte glycoprotein in incomplete Freund's adjuvant in three non-human primate species.J Neuroimmune Pharmacol. 2013 Dec;8(5):1251-64. doi: 10.1007/s11481-013-9487-z. Epub 2013 Jul 3. J Neuroimmune Pharmacol. 2013. PMID: 23821341 Free PMC article.
References
-
- Langermans JA, Andersen P, Van Soolingen D, et al. Divergent effect of bacillus Calmette–Guérin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc Natl Acad Sci USA. 2001;98:11497–502. - PMC - PubMed
-
- Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. - PubMed
-
- Jankovic V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

