The medial geniculate, not the amygdala, as the root of auditory fear conditioning
- PMID: 20466051
- PMCID: PMC2949681
- DOI: 10.1016/j.heares.2010.03.093
The medial geniculate, not the amygdala, as the root of auditory fear conditioning
Abstract
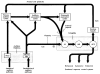
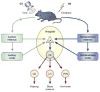
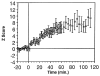
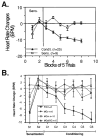
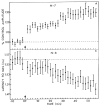
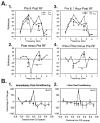
The neural basis of auditory fear conditioning (AFC) is almost universally believed to be the amygdala, where auditory fear memories are reputedly acquired and stored. This widely-accepted amygdala model holds that the auditory conditioned stimulus (CS) and the nociceptive unconditioned stimulus (US) first converge in the lateral nucleus of the amygdala (AL), and are projected independently to it from the medial division of the medial geniculate nucleus (MGm) and the adjacent posterior intralaminar nucleus (PIN), which serve merely as sensory relays. However, the four criteria that are used to support the AL model, (a) CS-US convergence, (b) associative plasticity, (c) LTP and (d) lesion-induced learning impairment, are also met by the MGm/PIN. Synaptic and molecular approaches supporting the AL also implicate the MGm/PIN. As both the AL and its preceding MGm/PIN are critically involved, we propose that the latter be considered the "root" of AFC.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Stimulation at a site of auditory-somatosensory convergence in the medial geniculate nucleus is an effective unconditioned stimulus for fear conditioning.Behav Neurosci. 1992 Jun;106(3):471-83. doi: 10.1037//0735-7044.106.3.471. Behav Neurosci. 1992. PMID: 1616614
-
Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli.J Neurosci. 1995 Mar;15(3 Pt 2):2312-27. doi: 10.1523/JNEUROSCI.15-03-02312.1995. J Neurosci. 1995. PMID: 7891169 Free PMC article.
-
Discriminative auditory fear learning requires both tuned and nontuned auditory pathways to the amygdala.J Neurosci. 2010 Jul 21;30(29):9782-7. doi: 10.1523/JNEUROSCI.1037-10.2010. J Neurosci. 2010. PMID: 20660260 Free PMC article.
-
Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning.Learn Mem. 2001 Sep-Oct;8(5):229-42. doi: 10.1101/lm.30901. Learn Mem. 2001. PMID: 11584069 Review.
-
Physiological memory in primary auditory cortex: characteristics and mechanisms.Neurobiol Learn Mem. 1998 Jul-Sep;70(1-2):226-51. doi: 10.1006/nlme.1998.3850. Neurobiol Learn Mem. 1998. PMID: 9753599 Review.
Cited by
-
Passive stimulation and behavioral training differentially transform temporal processing in the inferior colliculus and primary auditory cortex.J Neurophysiol. 2017 Jan 1;117(1):47-64. doi: 10.1152/jn.00392.2016. Epub 2016 Oct 12. J Neurophysiol. 2017. PMID: 27733594 Free PMC article.
-
Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall.Proc Natl Acad Sci U S A. 2013 Nov 5;110(45):18315-20. doi: 10.1073/pnas.1312508110. Epub 2013 Oct 22. Proc Natl Acad Sci U S A. 2013. PMID: 24151334 Free PMC article.
-
Biologically based neural circuit modelling for the study of fear learning and extinction.NPJ Sci Learn. 2016;1:16015. doi: 10.1038/npjscilearn.2016.15. Epub 2016 Nov 9. NPJ Sci Learn. 2016. PMID: 29541482 Free PMC article.
-
Adaptive categorization of sound frequency does not require the auditory cortex in rats.J Neurophysiol. 2015 Aug;114(2):1137-45. doi: 10.1152/jn.00124.2015. Epub 2015 Jul 8. J Neurophysiol. 2015. PMID: 26156379 Free PMC article.
-
A functional topography within the cholinergic basal forebrain for encoding sensory cues and behavioral reinforcement outcomes.Elife. 2021 Nov 25;10:e69514. doi: 10.7554/eLife.69514. Elife. 2021. PMID: 34821218 Free PMC article.
References
-
- Aitkin LM. Medial geniculate body of the cat: Responses to tonal stimuli of neurons in medial division. J Neurophysiol. 1973;36(2):275–283. - PubMed
-
- Aitkin LM, Webster WR. Medial geniculate body of the cat: Organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol. 1972;35(3):365–380. - PubMed
-
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3(1):74–79. - PubMed
-
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: Evidence for two largely segregated systems of connections. J Comp Neurol. 1980;194(3):663–701. - PubMed
-
- Apergis-Schoute AM, Debiec J, Doyère V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: A role for presynaptic plasticity in the fear system. J Neurosci. 2005;25(24):5730–5739. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

