Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies
- PMID: 20466091
- PMCID: PMC2869000
- DOI: 10.1016/j.ajhg.2010.04.006
Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies
Abstract
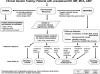
Chromosomal microarray (CMA) is increasingly utilized for genetic testing of individuals with unexplained developmental delay/intellectual disability (DD/ID), autism spectrum disorders (ASD), or multiple congenital anomalies (MCA). Performing CMA and G-banded karyotyping on every patient substantially increases the total cost of genetic testing. The International Standard Cytogenomic Array (ISCA) Consortium held two international workshops and conducted a literature review of 33 studies, including 21,698 patients tested by CMA. We provide an evidence-based summary of clinical cytogenetic testing comparing CMA to G-banded karyotyping with respect to technical advantages and limitations, diagnostic yield for various types of chromosomal aberrations, and issues that affect test interpretation. CMA offers a much higher diagnostic yield (15%-20%) for genetic testing of individuals with unexplained DD/ID, ASD, or MCA than a G-banded karyotype ( approximately 3%, excluding Down syndrome and other recognizable chromosomal syndromes), primarily because of its higher sensitivity for submicroscopic deletions and duplications. Truly balanced rearrangements and low-level mosaicism are generally not detectable by arrays, but these are relatively infrequent causes of abnormal phenotypes in this population (<1%). Available evidence strongly supports the use of CMA in place of G-banded karyotyping as the first-tier cytogenetic diagnostic test for patients with DD/ID, ASD, or MCA. G-banded karyotype analysis should be reserved for patients with obvious chromosomal syndromes (e.g., Down syndrome), a family history of chromosomal rearrangement, or a history of multiple miscarriages.
Copyright (c) 2010 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Shevell M., Ashwal S., Donley D., Flint J., Gingold M., Hirtz D., Majnemer A., Noetzel M., Sheth R.D. Practice parameter: evaluation of the child with global developmental delay: Report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology. 2003;60:367–380. - PubMed
-
- Autism and Developmental Monitoring Network Surveillance Year 2000 Principal Investigators Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill. Summ. 2007;56:1–11. - PubMed
-
- Newschaffer C.J., Croen L.A., Daniels J., Giarelli E., Grether J.K., Levy S.E., Mandell D.S., Miller L.A., Pinto-Martin J., Reaven J. The epidemiology of autism spectrum disorders. Annu. Rev. Public Health. 2007;28:235–258. - PubMed
-
- Moeschler J.B., Shevell M. Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics. 2006;117:2304–2316. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

