Direct interaction between XRCC1 and UNG2 facilitates rapid repair of uracil in DNA by XRCC1 complexes
- PMID: 20466601
- PMCID: PMC2901844
- DOI: 10.1016/j.dnarep.2010.04.002
Direct interaction between XRCC1 and UNG2 facilitates rapid repair of uracil in DNA by XRCC1 complexes
Abstract
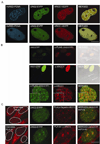
Uracil-DNA glycosylase, UNG2, interacts with PCNA and initiates post-replicative base excision repair (BER) of uracil in DNA. The DNA repair protein XRCC1 also co-localizes and physically interacts with PCNA. However, little is known about whether UNG2 and XRCC1 directly interact and participate in a same complex for repair of uracil in replication foci. Here, we examine localization pattern of these proteins in live and fixed cells and show that UNG2 and XRCC1 are likely in a common complex in replication foci. Using pull-down experiments we demonstrate that UNG2 directly interacts with the nuclear localization signal-region (NLS) of XRCC1. Western blot and functional analysis of immunoprecipitates from whole cell extracts prepared from S-phase enriched cells demonstrate the presence of XRCC1 complexes that contain UNG2 in addition to separate XRCC1 and UNG2 associated complexes with distinct repair features. XRCC1 complexes performed complete repair of uracil with higher efficacy than UNG2 complexes. Based on these results, we propose a model for a functional role of XRCC1 in replication associated BER of uracil.
2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





References
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. - PubMed
-
- Viswanathan A, You HJ, Doetsch PW. Phenotypic change caused by transcriptional bypass of uracil in nondividing cells. Science. 1999;284:159–162. - PubMed
-
- Akbari M, Pena-Diaz J, Andersen S, Liabakk NB, Otterlei M, Krokan HE. Extracts of proliferating and non-proliferating human cells display different base excision pathways and repair fidelity. DNA Repair (Amst.) 2009;8:834–843. - PubMed
-
- Sanderson RJ, Mosbaugh DW. Fidelity and mutational specificity of uracil-initiated base excision DNA repair synthesis in human glioblastoma cell extracts. J. Biol. Chem. 1998;273:24822–24831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

