Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy
- PMID: 20466848
- PMCID: PMC3075465
- DOI: 10.1161/CIRCHEARTFAILURE.109.900282
Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy
Abstract
Background: We have shown that glucagon-like peptide-1 (GLP-1[7-36] amide) stimulates myocardial glucose uptake in dilated cardiomyopathy (DCM) independent of an insulinotropic effect. The cellular mechanisms of GLP-1-induced myocardial glucose uptake are unknown.
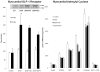
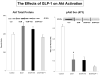
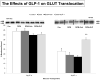
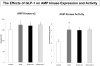
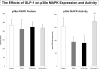
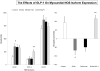
Methods and results: Myocardial substrates and glucoregulatory hormones were measured in conscious, chronically instrumented dogs at control (n=6), DCM (n=9) and DCM after treatment with a 48-hour infusion of GLP-1 (7-36) amide (n=9) or vehicle (n=6). GLP-1 receptors and cellular pathways implicated in myocardial glucose uptake were measured in sarcolemmal membranes harvested from the 4 groups. GLP-1 stimulated myocardial glucose uptake (DCM: 20+/-7 nmol/min/g; DCM+GLP-1: 61+/-12 nmol/min/g; P=0.001) independent of increased plasma insulin levels. The GLP-1 receptors were upregulated in the sarcolemmal membranes (control: 98+/-2 density units; DCM: 256+/-58 density units; P=0.046) and were expressed in their activated (65 kDa) form in DCM. The GLP-1-induced increases in myocardial glucose uptake did not involve adenylyl cyclase or Akt activation but was associated with marked increases in p38alpha MAP kinase activity (DCM+vehicle: 97+/-22 pmol ATP/mg/min; DCM+GLP-1: 170+/-36 pmol ATP/mg/min; P=0.051), induction of nitric oxide synthase 2 (DCM+vehicle: 151+/-13 density units; DCM+GLP-1: 306+/-12 density units; P=0.001), and GLUT-1 translocation (DCM+vehicle: 21+/-3% membrane bound; DCM+GLP-1: 39+/-3% membrane bound; P=0.005). The effects of GLP-1 on myocardial glucose uptake were blocked by pretreatment with the p38alpha MAP kinase inhibitor or the nonspecific nitric oxide synthase inhibitor nitro-l-arginine.
Conclusions: GLP-1 stimulates myocardial glucose uptake through a non-Akt-1-dependent mechanism by activating cellular pathways that have been identified in mediating chronic hibernation and the late phase of ischemic preconditioning.
Figures
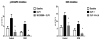
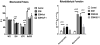
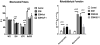
References
-
- Drucker DJ. Development of glucagon-like peptide-1-based pharmaceuticals as therapeutic agents for the treatment of diabetes. Curr Pharm Des. 2001;7:1399. - PubMed
-
- Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol. 2000;143:717–25. - PubMed
-
- Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001;56:377–99. - PubMed
-
- Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–206. - PubMed
-
- MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51:S434–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

