Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis
- PMID: 20466856
- PMCID: PMC2924221
- DOI: 10.1182/blood-2010-02-269746
Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis
Abstract
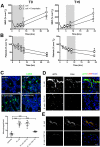
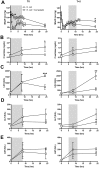
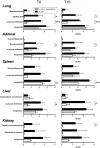
Severe sepsis leads to massive activation of coagulation and complement cascades that could contribute to multiple organ failure and death. To investigate the role of the complement and its crosstalk with the hemostatic system in the pathophysiology and therapeutics of sepsis, we have used a potent inhibitor (compstatin) administered early or late after Escherichia coli challenge in a baboon model of sepsis-induced multiple organ failure. Compstatin infusion inhibited sepsis-induced blood and tissue biomarkers of complement activation, reduced leucopenia and thrombocytopenia, and lowered the accumulation of macrophages and platelets in organs. Compstatin decreased the coagulopathic response by down-regulating tissue factor and PAI-1, diminished global blood coagulation markers (fibrinogen, fibrin-degradation products, APTT), and preserved the endothelial anticoagulant properties. Compstatin treatment also improved cardiac function and the biochemical markers of kidney and liver damage. Histologic analysis of vital organs collected from animals euthanized after 24 hours showed decreased microvascular thrombosis, improved vascular barrier function, and less leukocyte infiltration and cell death, all consistent with attenuated organ injury. We conclude that complement-coagulation interplay contributes to the progression of severe sepsis and blocking the harmful effects of complement activation products, especially during the organ failure stage of severe sepsis is a potentially important therapeutic strategy.
Figures




References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. - PubMed
-
- Taylor FB., Jr Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit Care Med. 2001;29(7 Suppl):S78–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

