Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish
- PMID: 20466977
- PMCID: PMC5497575
- DOI: 10.1161/ATVBAHA.110.203034
Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish
Abstract
Objective: To study whether Notch signaling, which regulates cell fate decisions and vessel morphogenesis, controls lymphatic development.
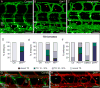
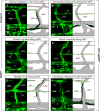
Methods and results: In zebrafish embryos, sprouts from the axial vein have lymphangiogenic potential because they give rise to the first lymphatics. Knockdown of delta-like-4 (Dll4) or its receptors Notch-1b or Notch-6 in zebrafish impaired lymphangiogenesis. Dll4/Notch silencing reduced the number of sprouts producing the string of parchordal lymphangioblasts; instead, sprouts connecting to the intersomitic vessels were formed. At a later phase, Notch silencing impaired navigation of lymphatic intersomitic vessels along their arterial templates.
Conclusions: These studies imply critical roles for Notch signaling in the formation and wiring of the lymphatic network.
Conflict of interest statement
Disclosure: The authors declare no competing financial interests.
Figures




Comment in
-
Notch leads lymphatics and links them to blood vessels.Arterioscler Thromb Vasc Biol. 2010 Sep;30(9):1682-3. doi: 10.1161/ATVBAHA.110.210633. Arterioscler Thromb Vasc Biol. 2010. PMID: 20720193 Free PMC article. No abstract available.
References
-
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. - PubMed
-
- Francois M, Koopman P, Beltrame M. SoxF genes: Key players in the development of the cardio-vascular system. Int J Biochem Cell Biol. 2010;42:445–448. - PubMed
-
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. - PubMed
-
- De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. - PubMed
-
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

