Chimeric antigen receptor-engineered T cells for immunotherapy of cancer
- PMID: 20467460
- PMCID: PMC2864912
- DOI: 10.1155/2010/956304
Chimeric antigen receptor-engineered T cells for immunotherapy of cancer
Abstract
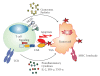
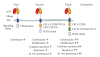
CD4+ and CD8+ T lymphocytes are powerful components of adaptive immunity, which essentially contribute to the elimination of tumors. Due to their cytotoxic capacity, T cells emerged as attractive candidates for specific immunotherapy of cancer. A promising approach is the genetic modification of T cells with chimeric antigen receptors (CARs). First generation CARs consist of a binding moiety specifically recognizing a tumor cell surface antigen and a lymphocyte activating signaling chain. The CAR-mediated recognition induces cytokine production and tumor-directed cytotoxicity of T cells. Second and third generation CARs include signal sequences from various costimulatory molecules resulting in enhanced T-cell persistence and sustained antitumor reaction. Clinical trials revealed that the adoptive transfer of T cells engineered with first generation CARs represents a feasible concept for the induction of clinical responses in some tumor patients. However, further improvement is required, which may be achieved by second or third generation CAR-engrafted T cells.
Figures

References
-
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. - PubMed
-
- Wang R-F. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends in Immunology. 2001;22(5):269–276. - PubMed
-
- Peggs KS. Adoptive T cell immunotherapy for cytomegalovirus. Expert Opinion on Biological Therapy. 2009;9(6):725–736. - PubMed
-
- Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annual Review of Immunology. 2007;25:243–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

