Isolation of nebulin from rabbit skeletal muscle and its interaction with actin
- PMID: 20467585
- PMCID: PMC2868979
- DOI: 10.1155/2010/108495
Isolation of nebulin from rabbit skeletal muscle and its interaction with actin
Abstract
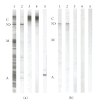
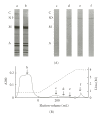
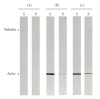
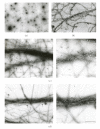
Nebulin is about 800 kDa filamentous protein that binds the entire thin filament of vertebrate skeletal muscle sarcomeres. Nebulin cannot be isolated from muscle except in a completely denatured form by direct solubilization of myofibrils with SDS because nebulin is hardly soluble under salt conditions. In the present study, nebulin was solubilized by a salt solution containing 1 M urea and purified by DEAE-Toyopearl column chromatography via 4 M urea elution. Rotary-shadowed images of nebulin showed entangled knit-like particles, about 20 nm in diameter. The purified nebulin bound to actin filaments to form loose bundles. Nebulin was confirmed to bind actin, alpha-actinin, beta-actinin, and tropomodulin, but not troponin or tropomyosin. The data shows that full-length nebulin can be also obtained in a functional and presumably native form, verified by data from experiments using recombinant subfragments.
Figures



Similar articles
-
The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments.J Biol Chem. 2001 Jan 5;276(1):583-92. doi: 10.1074/jbc.M005693200. J Biol Chem. 2001. PMID: 11016930
-
Expression and purification of large nebulin fragments and their interaction with actin.Biophys J. 1998 Jan;74(1):349-59. doi: 10.1016/S0006-3495(98)77792-6. Biophys J. 1998. PMID: 9449335 Free PMC article.
-
Determination of the protein components of native thin filaments isolated from natural actomyosin: nebulin and alpha-actinin are associated with actin filaments.J Biochem. 1995 Aug;118(2):422-7. doi: 10.1093/oxfordjournals.jbchem.a124924. J Biochem. 1995. PMID: 8543580
-
Defining actin filament length in striated muscle: rulers and caps or dynamic stability?Annu Rev Cell Dev Biol. 1998;14:487-525. doi: 10.1146/annurev.cellbio.14.1.487. Annu Rev Cell Dev Biol. 1998. PMID: 9891791 Review.
-
New insights into the structural roles of nebulin in skeletal muscle.J Biomed Biotechnol. 2010;2010:968139. doi: 10.1155/2010/968139. Epub 2010 Jun 1. J Biomed Biotechnol. 2010. PMID: 20589077 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of the Deregulation of Muscle Contraction Induced by the R90P Mutation in Tpm3.12 and the Weakening of This Effect by BDM and W7.Int J Mol Sci. 2021 Jun 12;22(12):6318. doi: 10.3390/ijms22126318. Int J Mol Sci. 2021. PMID: 34204776 Free PMC article.
-
Nebulin interactions with actin and tropomyosin are altered by disease-causing mutations.Skelet Muscle. 2014 Aug 1;4:15. doi: 10.1186/2044-5040-4-15. eCollection 2014. Skelet Muscle. 2014. PMID: 25110572 Free PMC article.
-
Nebulin, a multi-functional giant.J Exp Biol. 2016 Jan;219(Pt 2):146-52. doi: 10.1242/jeb.126383. J Exp Biol. 2016. PMID: 26792324 Free PMC article. Review.
-
Nebulin: big protein with big responsibilities.J Muscle Res Cell Motil. 2020 Mar;41(1):103-124. doi: 10.1007/s10974-019-09565-3. Epub 2020 Jan 25. J Muscle Res Cell Motil. 2020. PMID: 31982973 Free PMC article. Review.
-
Tropomodulins: pointed-end capping proteins that regulate actin filament architecture in diverse cell types.Cytoskeleton (Hoboken). 2012 Jun;69(6):337-70. doi: 10.1002/cm.21031. Epub 2012 May 4. Cytoskeleton (Hoboken). 2012. PMID: 22488942 Free PMC article. Review.
References
-
- Labeit S, Kolmerer B. The complete primary structure of human nebulin and its correlation to muscle structure. Journal of Molecular Biology. 1995;248(2):308–315. - PubMed
-
- Labeit S, Gibson T, Lakey A, et al. Evidence that nebulin is a protein-ruler in muscle thin filaments. FEBS Letters. 1991;282(2):313–316. - PubMed
-
- Wang K. Purification of titin and nebulin. Methods in Enzymology B. 1982;85:264–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
