Simulating dissolution of intravitreal triamcinolone acetonide suspensions in an anatomically accurate rabbit eye model
- PMID: 20467888
- PMCID: PMC2896643
- DOI: 10.1007/s11095-010-0163-1
Simulating dissolution of intravitreal triamcinolone acetonide suspensions in an anatomically accurate rabbit eye model
Abstract
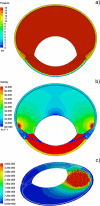
Purpose: A computational fluid dynamics (CFD) study examined the impact of particle size on dissolution rate and residence of intravitreal suspension depots of Triamcinolone Acetonide (TAC).
Methods: A model for the rabbit eye was constructed using insights from high-resolution NMR imaging studies (Sawada 2002). The current model was compared to other published simulations in its ability to predict clearance of various intravitreally injected materials. Suspension depots were constructed explicitly rendering individual particles in various configurations: 4 or 16 mg drug confined to a 100 microL spherical depot, or 4 mg exploded to fill the entire vitreous. Particle size was reduced systematically in each configuration. The convective diffusion/dissolution process was simulated using a multiphase model.
Results: Release rate became independent of particle diameter below a certain value. The size-independent limits occurred for particle diameters ranging from 77 to 428 microM depending upon the depot configuration. Residence time predicted for the spherical depots in the size-independent limit was comparable to that observed in vivo.
Conclusions: Since the size-independent limit was several-fold greater than the particle size of commercially available pharmaceutical TAC suspensions, differences in particle size amongst such products are predicted to be immaterial to their duration or performance.
Figures













References
-
- Sebag J. The vitreous: structure, function and pathobiology. New York: Springer-Verlag; 1989.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

