Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors
- PMID: 20470445
- PMCID: PMC2885345
- DOI: 10.1186/1476-4598-9-107
Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors
Abstract
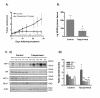
Background: The orally active quinoline-3-carboxamide tasquinimod [ABR-215050; CAS number 254964-60-8), which currently is in a phase II-clinical trial in patients against metastatic prostate cancer, exhibits anti-tumor activity via inhibition of tumor angiogenesis in human and rodent tumors. To further explore the mode of action of tasquinimod, in vitro and in vivo experiments with gene microarray analysis were performed using LNCaP prostate tumor cells. The array data were validated by real-time semiquantitative reversed transcriptase polymerase chain reaction (sqRT-PCR) and protein expression techniques.
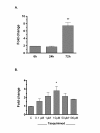
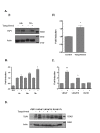
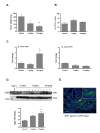
Results: One of the most significant differentially expressed genes both in vitro and in vivo after exposure to tasquinimod, was thrombospondin-1 (TSP1). The up-regulation of TSP1 mRNA in LNCaP tumor cells both in vitro and in vivo correlated with an increased expression and extra cellular secretion of TSP1 protein. When nude mice bearing CWR-22RH human prostate tumors were treated with oral tasquinimod, there was a profound growth inhibition, associated with an up-regulation of TSP1 and a down- regulation of HIF-1 alpha protein, androgen receptor protein (AR) and glucose transporter-1 protein within the tumor tissue. Changes in TSP1 expression were paralleled by an anti-angiogenic response, as documented by decreased or unchanged tumor tissue levels of VEGF (a HIF-1 alpha down stream target) in the tumors from tasquinimod treated mice.
Conclusions: We conclude that tasquinimod-induced up-regulation of TSP1 is part of a mechanism involving down-regulation of HIF1alpha and VEGF, which in turn leads to reduced angiogenesis via inhibition of the "angiogenic switch", that could explain tasquinimods therapeutic potential.
Figures

References
-
- Dalrymple SL, Becker RE, Isaacs JT. The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate. 2007;67:790–797. doi: 10.1002/pros.20573. - DOI - PubMed
-
- Isaacs JT, Pili R, Qian DZ, Dalrymple SL, Garrison JB, Kyprianou N, Bjork A, Olsson A, Leanderson T. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate. 2006;66:1768–1778. doi: 10.1002/pros.20509. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

