The neuro-ophthalmology of mitochondrial disease
- PMID: 20471050
- PMCID: PMC2989385
- DOI: 10.1016/j.survophthal.2009.10.002
The neuro-ophthalmology of mitochondrial disease
Abstract
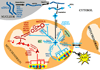
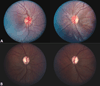
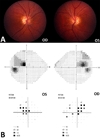
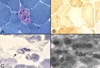
Mitochondrial diseases frequently manifest neuro-ophthalmologic symptoms and signs. Because of the predilection of mitochondrial disorders to involve the optic nerves, extraocular muscles, retina, and even the retrochiasmal visual pathways, the ophthalmologist is often the first physician to be consulted. Disorders caused by mitochondrial dysfunction can result from abnormalities in either the mitochondrial DNA or in nuclear genes which encode mitochondrial proteins. Inheritance of these mutations will follow patterns specific to their somatic or mitochondrial genetics. Genotype-phenotype correlations are inconstant, and considerable overlap may occur among these syndromes. The diagnostic approach to the patient with suspected mitochondrial disease entails a detailed personal and family history, careful ophthalmic, neurologic, and systemic examination, directed investigations, and attention to potentially life-threatening sequelae. Although curative treatments for mitochondrial disorders are currently lacking, exciting research advances are being made, particularly in the area of gene therapy. Leber hereditary optic neuropathy, with its window of opportunity for timely intervention and its accessibility to directed therapy, offers a unique model to study future therapeutic interventions. Most patients and their relatives benefit from informed genetic counseling.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures







References
-
- Abe K, Yoshimura H, Tanaka H, et al. Comparison of conventional and diffusion-weighted MRI and proton MR spectroscopy in patients with mitochondrial encephalopathy, lactic acidosis, and stroke-like events. Neuroradiology. 2004;46:113–117. - PubMed
-
- Adjadj E, Mansouri K, Borruat FX. Mitochondrial DNA (mtDNA) A3243G mutation associated with an annular perimacular retinal atrophy. Klin Monatsbl Augenheilkd. 2008;225:462–464. - PubMed
-
- Ahn J, Kim NJ, Choung HK, et al. Frontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegia. Br J Ophthalmol. 2008;92:1685–1688. - PubMed
-
- Aijaz S, Erskine L, Jeffery G, et al. Developmental expression profile of the optic atrophy gene product: OPA1 is not localized exclusively in the mammalian retinal ganglion cell layer. Invest Ophthalmol Visual Sci. 2004;45:1667–1673. - PubMed
-
- Amati-Bonneau P, Milea D, Bonneau D, et al. OPA1-associated disorders: phenotypes and pathophysiology. Int J Biochem Cell Biol. 2009;41:1855–1865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

