Rapid expansion and functional divergence of subtelomeric gene families in yeasts
- PMID: 20471265
- PMCID: PMC2877759
- DOI: 10.1016/j.cub.2010.04.027
Rapid expansion and functional divergence of subtelomeric gene families in yeasts
Abstract
Background: Subtelomeres, regions proximal to telomeres, exhibit characteristics unique to eukaryotic genomes. Genes residing in these loci are subject to epigenetic regulation and elevated rates of both meiotic and mitotic recombination. However, most genome sequences do not contain assembled subtelomeric sequences, and, as a result, subtelomeres are often overlooked in comparative genomics.
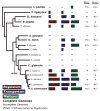
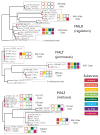
Results: We studied the evolution and functional divergence of subtelomeric gene families in the yeast lineage. Our computational results show that subtelomeric families are evolving and expanding much faster than families that do not contain subtelomeric genes. Focusing on three related subtelomeric MAL gene families involved in disaccharide metabolism that show typical patterns of rapid expansion and evolution, we show experimentally how frequent duplication events followed by functional divergence yield novel alleles that allow the metabolism of different carbohydrates.
Conclusions: Taken together, our computational and experimental analyses show that the extraordinary instability of eukaryotic subtelomeres supports rapid adaptation to novel niches by promoting gene recombination and duplication followed by functional divergence of the alleles.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures




References
-
- Pryde FE, Gorham HC, Louis EJ. Chromosome ends: all the same under their caps. Curr Opin Genet Dev. 1997;7:822–828. - PubMed
-
- Cohn M, Liti G, Barton DBH. Telomeres in fungi. Top Curr Genet. 2006;15:101–130.
-
- Mefford H, Trask B. The complex structure and dynamic evolution of human subtelomeres. Nat Rev Genet. 2002;3:91–102. - PubMed
-
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

