Neck linker length determines the degree of processivity in kinesin-1 and kinesin-2 motors
- PMID: 20471270
- PMCID: PMC2882250
- DOI: 10.1016/j.cub.2010.03.065
Neck linker length determines the degree of processivity in kinesin-1 and kinesin-2 motors
Abstract
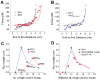
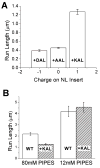
Defining the mechanical and biochemical determinates of kinesin processivity is important for understanding how diverse kinesins are tuned for specific cellular functions. Because transmission of mechanical forces through the 14-18 amino acid neck linker domain underlies coordinated stepping, we investigated the role of neck linker length, charge, and structure in kinesin-1 and kinesin-2 motor behavior. For optimum comparison with kinesin-1, the KIF3A head and neck linker of kinesin-2 were fused to the kinesin-1 neck coil and rod. Extending the 14-residue kinesin-1 neck linker reduced processivity, and shortening the 17-residue kinesin-2 neck linker enhanced processivity. When a proline in the kinesin-2 neck linker was replaced, kinesin-1 and kinesin-2 run lengths scaled identically with neck linker length, despite moving at different speeds. In low-ionic-strength buffer, charge had a dominant effect on motor processivity, which resolves ongoing controversy regarding the effect of neck linker length on kinesin processivity. From stochastic simulations, the results are best explained by neck linker extension slowing strain-dependent detachment of the rear head along with diminishing strain-dependent inhibition of ATP binding. These results help delineate how interhead strain maximizes stepping and suggest that less processive kinesins are tuned to coordinate with other motors differently than the maximally processive kinesin-1.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. - PubMed
-
- Hackney DD, Stock MF, Moore J, Patterson RA. Modulation of kinesin half-site ADP release and kinetic processivity by a spacer between the head groups. Biochemistry. 2003;42:12011–12018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

