SIRT1 and p53, effect on cancer, senescence and beyond
- PMID: 20471503
- PMCID: PMC2989880
- DOI: 10.1016/j.bbapap.2010.05.002
SIRT1 and p53, effect on cancer, senescence and beyond
Abstract
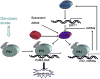
NAD(+)-dependent Class III histone deacetylase SIRT1 is a multiple function protein critically involved in stress responses, cellular metabolism and aging through deacetylating a variety of substrates including p53, forkhead-box transcription factors, PGC-1alpha, NF-kappaB, Ku70 and histones. The first discovered non-histone target of SIRT1, p53, is suggested to play a central role in SIRT1-mediated functions in tumorigenesis and senescence. SIRT1 was originally considered to be a potential tumor promoter since it negatively regulates the tumor suppressor p53 and other tumor suppressors. There is new evidence that SIRT1 acts as a tumor suppressor based on its role in negatively regulating beta-catenin and survivin. This review provides an overview of current knowledge of SIRT1-p53 signaling and controversies regarding the functions of SIRT1 in tumorigenesis.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. - PubMed
-
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. - PubMed
-
- Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408:377–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

