Chaperone networks: tipping the balance in protein folding diseases
- PMID: 20472062
- PMCID: PMC3429345
- DOI: 10.1016/j.nbd.2010.05.007
Chaperone networks: tipping the balance in protein folding diseases
Abstract
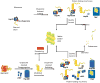
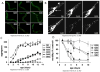
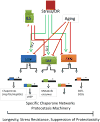
Adult-onset neurodegeneration and other protein conformational diseases are associated with the appearance, persistence, and accumulation of misfolded and aggregation-prone proteins. To protect the proteome from long-term damage, the cell expresses a highly integrated protein homeostasis (proteostasis) machinery to ensure that proteins are properly expressed, folded, and cleared, and to recognize damaged proteins. Molecular chaperones have a central role in proteostasis as they have been shown to be essential to prevent the accumulation of alternate folded proteotoxic states as occurs in protein conformation diseases exemplified by neurodegeneration. Studies using invertebrate models expressing proteins associated with Huntington's disease, Alzheimer's disease, ALS, and Parkinson's disease have provided insights into the genetic networks and stress signaling pathways that regulate the proteostasis machinery to prevent cellular dysfunction, tissue pathology, and organismal failure. These events appear to be further amplified by aging and provide evidence that age-related failures in proteostasis may be a common element in many diseases.
(c) 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–30. - PubMed
-
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. - PubMed
-
- Balch WE, et al. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

