Pegaptanib sodium as maintenance therapy in neovascular age-related macular degeneration: the LEVEL study
- PMID: 20472746
- PMCID: PMC2991041
- DOI: 10.1136/bjo.2009.174946
Pegaptanib sodium as maintenance therapy in neovascular age-related macular degeneration: the LEVEL study
Abstract
Aim: To assess the efficacy of pegaptanib as maintenance therapy in neovascular age-related macular degeneration (NV-AMD) patients after induction therapy.
Methods: A phase IV, prospective, open-label, uncontrolled exploratory study including subjects with subfoveal NV-AMD who had had one to three induction treatments 30-120 days before entry and showed investigator-determined clinical/anatomical NV-AMD improvement. Lesions in the study eye were: any subtype, 12 or fewer disc areas; postinduction centre point thickness (CPT) 275 μm or less or thinning of 100 μm or more (optical coherence tomography); visual acuity (VA) 20/20-20/400. Intravitreal pegaptanib 0.3 mg was administered as maintenance every 6 weeks for 48 weeks with follow-up to week 54. Booster treatment additional unscheduled treatment for wet age-related macular degeneration, was allowed in the study eye at the investigators' discretion for clinical deterioration.
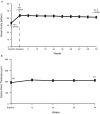
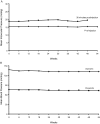
Results: Of 568 enrolled subjects, 86% completed 1 year of pegaptanib. Mean VA improvement during induction (49.6 to 65.5 letters) was well preserved (54-week mean 61.8 letters). Mean CPT was relatively stable during maintenance (20 μm increase during the study). Fifty per cent did not receive unscheduled booster treatment to week 54; 46% did have one such booster (mean 147 days after maintenance initiation).
Conclusions: An induction-maintenance strategy, using non-selective then selective vascular endothelial growth factor (VEGF) inhibitors, could be considered for NV-AMD. This approach may have particular relevance for patients with systemic comorbidities who require long-term anti-VEGF therapy for NV-AMD.
Trial registration: ClinicalTrials.gov NCT00354445.
Conflict of interest statement
Figures

References
-
- Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805–16 - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–44 - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31 - PubMed
-
- Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother 2007;41:614–25 - PubMed
-
- Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859–70 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
