The response regulator SprE (RssB) is required for maintaining poly(A) polymerase I-degradosome association during stationary phase
- PMID: 20472786
- PMCID: PMC2897334
- DOI: 10.1128/JB.00300-10
The response regulator SprE (RssB) is required for maintaining poly(A) polymerase I-degradosome association during stationary phase
Abstract
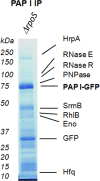
Poly(A) polymerase I (PAP I) is the enzyme responsible for the addition of poly(A) tails onto RNA molecules in Escherichia coli. Polyadenylation is believed to facilitate the destruction of such RNAs by the mRNA degradosome. Recently, it was discovered that the stationary-phase regulatory protein SprE (RssB) has a second function in the control of polyadenylation that is distinct from its known function in the regulated proteolysis of RpoS. In the work presented herein, we used a targeted proteomic approach to further investigate SprE's involvement in the polyadenylation pathway. Specifically, we used cryogenic cell lysis, immunopurifications on magnetic beads, and mass spectrometry to identify interacting partners of PAP I-green fluorescent protein. We provide the first in vivo evidence that PAP I interacts with the mRNA degradosome during both exponential and stationary phases and find that the degradosome can contain up to 10 different proteins under certain conditions. Moreover, we demonstrate that the majority of these PAP I interactions are formed via protein-protein interactions and that SprE plays an important role in the maintenance of the PAP I-degradosome association during stationary phase.
Figures




Similar articles
-
The response regulator SprE (RssB) modulates polyadenylation and mRNA stability in Escherichia coli.J Bacteriol. 2009 Nov;191(22):6812-21. doi: 10.1128/JB.00870-09. Epub 2009 Sep 18. J Bacteriol. 2009. PMID: 19767441 Free PMC article.
-
RNase E-based degradosome modulates polyadenylation of mRNAs after Rho-independent transcription terminators in Escherichia coli.Mol Microbiol. 2016 Aug;101(4):645-55. doi: 10.1111/mmi.13413. Epub 2016 Jun 16. Mol Microbiol. 2016. PMID: 27145979 Free PMC article.
-
RNA stability is regulated by both RNA polyadenylation and ATP levels, linking RNA and energy metabolisms in Escherichia coli.mBio. 2025 Jan 8;16(1):e0268024. doi: 10.1128/mbio.02680-24. Epub 2024 Nov 29. mBio. 2025. PMID: 39611689 Free PMC article.
-
Polyadenylation of mRNA in bacteria.Microbiology (Reading). 1996 Nov;142 ( Pt 11):3125-33. doi: 10.1099/13500872-142-11-3125. Microbiology (Reading). 1996. PMID: 8969510 Review. No abstract available.
-
Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli.Mol Microbiol. 1996 Sep;21(5):887-93. doi: 10.1046/j.1365-2958.1996.511405.x. Mol Microbiol. 1996. PMID: 8885260 Review.
Cited by
-
Immunoisolation of protein complexes from Xenopus.Methods Mol Biol. 2012;917:369-90. doi: 10.1007/978-1-61779-992-1_21. Methods Mol Biol. 2012. PMID: 22956099 Free PMC article.
-
Initiation of mRNA decay in bacteria.Cell Mol Life Sci. 2014 May;71(10):1799-828. doi: 10.1007/s00018-013-1472-4. Epub 2013 Sep 25. Cell Mol Life Sci. 2014. PMID: 24064983 Free PMC article. Review.
-
RpoS and the bacterial general stress response.Microbiol Mol Biol Rev. 2024 Mar 27;88(1):e0015122. doi: 10.1128/mmbr.00151-22. Epub 2024 Feb 27. Microbiol Mol Biol Rev. 2024. PMID: 38411096 Free PMC article. Review.
-
Structural elucidation of a novel mechanism for the bacteriophage-based inhibition of the RNA degradosome.Elife. 2016 Jul 22;5:e16413. doi: 10.7554/eLife.16413. Elife. 2016. PMID: 27447594 Free PMC article.
-
RNase R is associated in a functional complex with the RhpA DEAD-box RNA helicase in Helicobacter pylori.Nucleic Acids Res. 2021 May 21;49(9):5249-5264. doi: 10.1093/nar/gkab283. Nucleic Acids Res. 2021. PMID: 33893809 Free PMC article.
References
-
- Andrade, J. M., F. Cairrao, and C. M. Arraiano. 2006. RNase R affects gene expression in stationary phase: regulation of ompA. Mol. Microbiol. 60:219-228. - PubMed
-
- Blum, E., A. J. Carpousis, and C. F. Higgins. 1999. Polyadenylation promotes degradation of 3′-structured RNA by the Escherichia coli mRNA degradosome in vitro. J. Biol. Chem. 274:4009-4016. - PubMed
-
- Blum, E., B. Py, A. J. Carpousis, and C. F. Higgins. 1997. Polyphosphate kinase is a component of the Escherichia coli RNA degradosome. Mol. Microbiol. 26:387-398. - PubMed
-
- Brancia, F. L., S. G. Oliver, and S. J. Gaskell. 2000. Improved matrix-assisted laser desorption/ionization mass spectrometric analysis of tryptic hydrolysates of proteins following guanidination of lysine-containing peptides. Rapid Commun. Mass Spectrom. 14:2070-2073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

