Differential effects of myopathy-associated caveolin-3 mutants on growth factor signaling
- PMID: 20472890
- PMCID: PMC2893669
- DOI: 10.2353/ajpath.2010.090741
Differential effects of myopathy-associated caveolin-3 mutants on growth factor signaling
Abstract
Caveolin-3 is an important scaffold protein of cholesterol-rich caveolae. Mutations of caveolin-3 cause hereditary myopathies that comprise remarkably different pathologies. Growth factor signaling plays an important role in muscle physiology; it is influenced by caveolins and cholesterol-rich rafts and might thus be affected by caveolin-3 dysfunction. Prompted by the observation of a marked chronic peripheral neuropathy in a patient suffering from rippling muscle disease due to the R26Q caveolin-3 mutation and because TrkA is expressed by neuronal cells and skeletal muscle fibers, we performed a detailed comparative study on the effect of pathogenic caveolin-3 mutants on the signaling and trafficking of the TrkA nerve growth factor receptor and, for comparison, of the epidermal growth factor receptor. We found that the R26Q mutant slightly and the P28L strongly reduced nerve growth factor signaling in TrkA-transfected cells. Surface biotinylation experiments revealed that the R26Q caveolin-3 mutation markedly reduced the internalization of TrkA, whereas the P28L did not. Moreover, P28L expression led to increased, whereas R26Q expression decreased, epidermal growth factor signaling. Taken together, we found differential effects of the R26Q and P28L caveolin-3 mutants on growth factor signaling. Our findings are of clinical interest because they might help explain the remarkable differences in the degree of muscle lesions caused by caveolin-3 mutations and also the co-occurrence of peripheral neuropathy in the R26Q caveolinopathy case presented.
Figures

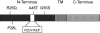








References
-
- Lisanti MP, Sargiacomo M, Scherer PE. Purification of caveolae-derived membrane microdomains containing lipid-anchored signaling molecules, such as GPI-anchored proteins. H-Ras, Src-family tyrosine kinases, eNOS, and G-protein alpha-, beta-, and gamma-subunits. Methods Mol Biol. 1999;116:51–60. - PubMed
-
- Way M, Parton RG. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 1996;378:108–112. - PubMed
-
- Vorgerd M, Ricker K, Ziemssen F, Kress W, Goebel HH, Nix WA, Kubisch C, Schoser BG, Mortier W. A sporadic case of rippling muscle disease caused by a de novo caveolin-3 mutation. Neurology. 2001;57:2273–2277. - PubMed
-
- Herrmann R, Straub V, Blank M, Kutzick C, Franke N, Jacob EN, Lenard HG, Kroger S, Voit T. Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum Mol Genet. 2000;9:2335–2340. - PubMed
-
- Sotgia F, Woodman SE, Bonuccelli G, Capozza F, Minetti C, Scherer PE, Lisanti MP. Phenotypic behavior of caveolin-3 R26Q, a mutant associated with hyperCKemia, distal myopathy, and rippling muscle disease. Am J Physiol Cell Physiol. 2003;285:C1150–C1160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

