Bisphosphonates cause osteonecrosis of the jaw-like disease in mice
- PMID: 20472893
- PMCID: PMC2893671
- DOI: 10.2353/ajpath.2010.090592
Bisphosphonates cause osteonecrosis of the jaw-like disease in mice
Abstract
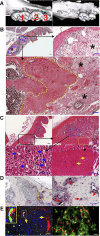
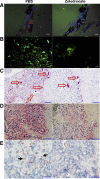
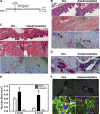
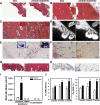
Bisphosphonate-associated osteonecrosis of the jaw (BONJ) is a morbid bone disease linked to long-term bisphosphonate use. Despite its broad health impact, mechanistic study is lacking. In this study, we have established a mouse model of BONJ-like disease based on the equivalent clinical regimen in myeloma patients, a group associated with high risk of BONJ. We demonstrate that the murine BONJ-like disease recapitulates major clinical and radiographical manifestations of the human disease, including characteristic features of osseous sclerosis, sequestra, avascular, and radiopaque alveolar bone in the jaw that persists beyond a normal course of wound healing following tooth extraction. We find that long-term administration of bisphosphonates results in an increase in the size and number of osteoclasts and the formation of giant osteoclast-like cells within the alveolar bone. We show that the development of necrotic bone and impaired soft tissue healing in our mouse model is dependent on long-term use of high-dose bisphosphonates, immunosuppressive and chemotherapy drugs, as well as mechanical trauma. Most importantly, we demonstrate that bisphosphonate is the major cause of BONJ-like disease in mice, mediated in part by its ability to suppress osseous angiogenesis and bone remodeling. The availability of this novel mouse model of BONJ-like disease will help elucidate the pathophysiology of BONJ and ultimately develop novel approaches for prevention and treatment of human BONJ.
Figures
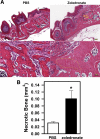
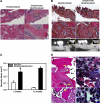
References
-
- Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. - PubMed
-
- Ruggiero SL, Woo SB. Bisphosphonate-related osteonecrosis of the jaws. Dent Clin North Am. 2008;52:111–128. - PubMed
-
- Kuehn BM. Reports of adverse events from bone drugs prompt caution. JAMA. 2006;295:2833–2836. - PubMed
-
- Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. - PubMed
-
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

