Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice
- PMID: 20472897
- PMCID: PMC2893691
- DOI: 10.2353/ajpath.2010.090480
Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice
Abstract
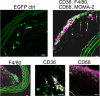
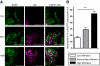
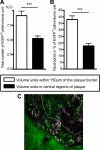
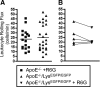
Inflammation and activation of immune cells are key mechanisms in the development of atherosclerosis. Previous data indicate important roles for monocytes and T-lymphocytes in lesions. However, recent data suggest that neutrophils also may be of importance in atherogenesis. Here, we use apolipoprotein E (ApoE)-deficient mice with fluorescent neutrophils and monocytes (ApoE(-/-)/Lys(EGFP/EGFP) mice) to specifically study neutrophil presence and recruitment in atherosclerotic lesions. We show by flow cytometry and confocal microscopy that neutrophils make up for 1.8% of CD45(+) leukocytes in the aortic wall of ApoE(-/-)/Lys(EGFP/EGFP) mice and that their contribution relative to monocyte/macrophages within lesions is approximately 1:3. However, neutrophils accumulate at sites of monocyte high density, preferentially in shoulder regions of lesions, and may even outnumber monocyte/macrophages in these areas. Furthermore, intravital microscopy established that a majority of leukocytes interacting with endothelium on lesion shoulders are neutrophils, suggesting a significant recruitment of these cells to plaque. These data demonstrate neutrophilic granulocytes as a major cellular component of atherosclerotic lesions in ApoE(-/-) mice and call for further study on the roles of these cells in atherogenesis.
Figures

References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. - PubMed
-
- Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. - PubMed
-
- Edfeldt K, Agerberth B, Rottenberg ME, Gudmundsson GH, Wang XB, Mandal K, Xu Q, Yan ZQ. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1551–1557. - PubMed
-
- van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR-/- mice. Arterioscler Thromb Vasc Biol. 2008;28:84–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

