Serine 34 phosphorylation of rho guanine dissociation inhibitor (RhoGDIalpha) links signaling from conventional protein kinase C to RhoGTPase in cell adhesion
- PMID: 20472934
- PMCID: PMC2906322
- DOI: 10.1074/jbc.M109.098129
Serine 34 phosphorylation of rho guanine dissociation inhibitor (RhoGDIalpha) links signaling from conventional protein kinase C to RhoGTPase in cell adhesion
Abstract
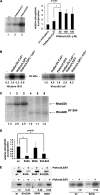
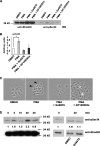
Conventional protein kinase C (PKC) isoforms are essential serine/threonine kinases regulating many signaling networks. At cell adhesion sites, PKCalpha can impact the actin cytoskeleton through its influence on RhoGTPases, but the intermediate steps are not well known. One important regulator of RhoGTPase function is the multifunctional guanine nucleotide dissociation inhibitor RhoGDIalpha that sequesters several related RhoGTPases in an inactive form, but it may also target them through interactions with actin-associated proteins. Here, it is demonstrated that conventional PKC phosphorylates RhoGDIalpha on serine 34, resulting in a specific decrease in affinity for RhoA but not Rac1 or Cdc42. The mechanism of RhoGDIalpha phosphorylation is distinct, requiring the kinase and phosphatidylinositol 4,5-bisphosphate, consistent with recent evidence that the inositide can activate, localize, and orient PKCalpha in membranes. Phosphospecific antibodies reveal endogenous phosphorylation in several cell types that is sensitive to adhesion events triggered, for example, by hepatocyte growth factor. Phosphorylation is also sensitive to PKC inhibition. Together with fluorescence resonance energy transfer microscopy sensing GTP-RhoA levels, the data reveal a common pathway in cell adhesion linking two essential mediators, conventional PKC and RhoA.
Figures


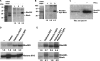

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

